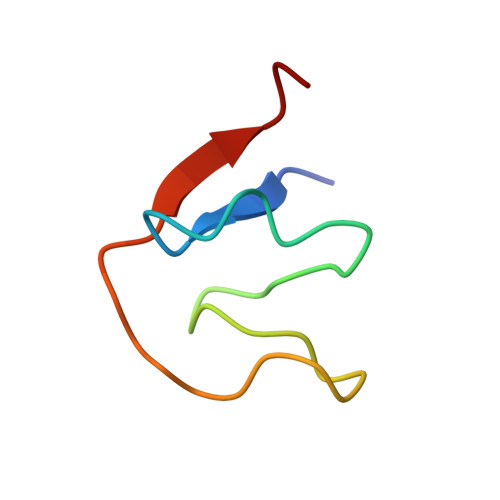
Structure of a Zinc-binding Domain in the Junin Virus Envelope Glycoprotein.
Briknarova, K., Thomas, C.J., York, J., Nunberg, J.H.(2011) J Biological Chem 286: 1528-1536
- PubMed: 21068387
- DOI: https://doi.org/10.1074/jbc.M110.166025
- Primary Citation of Related Structures:
2L0Z - PubMed Abstract:
Arenaviruses cause acute hemorrhagic fevers with high mortality. Entry of the virus into the host cell is mediated by the viral envelope glycoprotein, GPC. In contrast to other class I viral envelope glycoproteins, the mature GPC complex contains a cleaved stable signal peptide (SSP) in addition to the canonical receptor-binding (G1) and transmembrane fusion (G2) subunits. SSP is critical for intracellular transport of the GPC complex to the cell surface and for its membrane-fusion activity. Previous studies have suggested that SSP is retained in GPC through interaction with a zinc-binding domain (ZBD) in the cytoplasmic tail of G2. Here we used NMR spectroscopy to determine the structure of Junín virus (JUNV) ZBD (G2 residues 445-485) and investigate its interaction with a conserved Cys residue (Cys-57) in SSP. We show that JUNV ZBD displays a novel fold containing two zinc ions. One zinc ion is coordinated by His-447, His-449, Cys-455, and His-485. The second zinc ion is coordinated by His-459, Cys-467, and Cys-469 and readily accepts Cys-57 from SSP as the fourth ligand. Our studies describe the structural basis for retention of the unique SSP subunit and suggest a mechanism whereby SSP is positioned in the GPC complex to modulate pH-dependent membrane fusion.
- Department of Chemistry and Biochemistry, University of Montana, Missoula, Montana 59812, USA. klara.briknarova@umontana.edu
Organizational Affiliation: