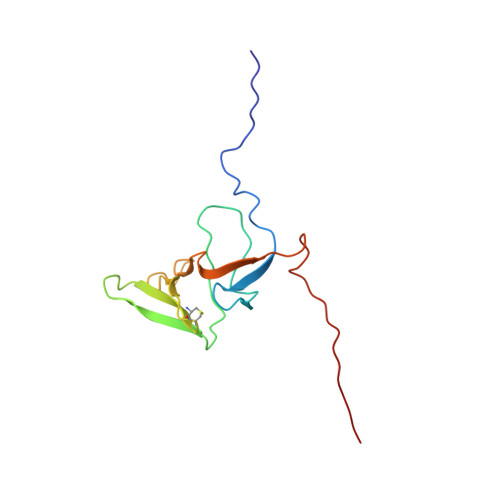
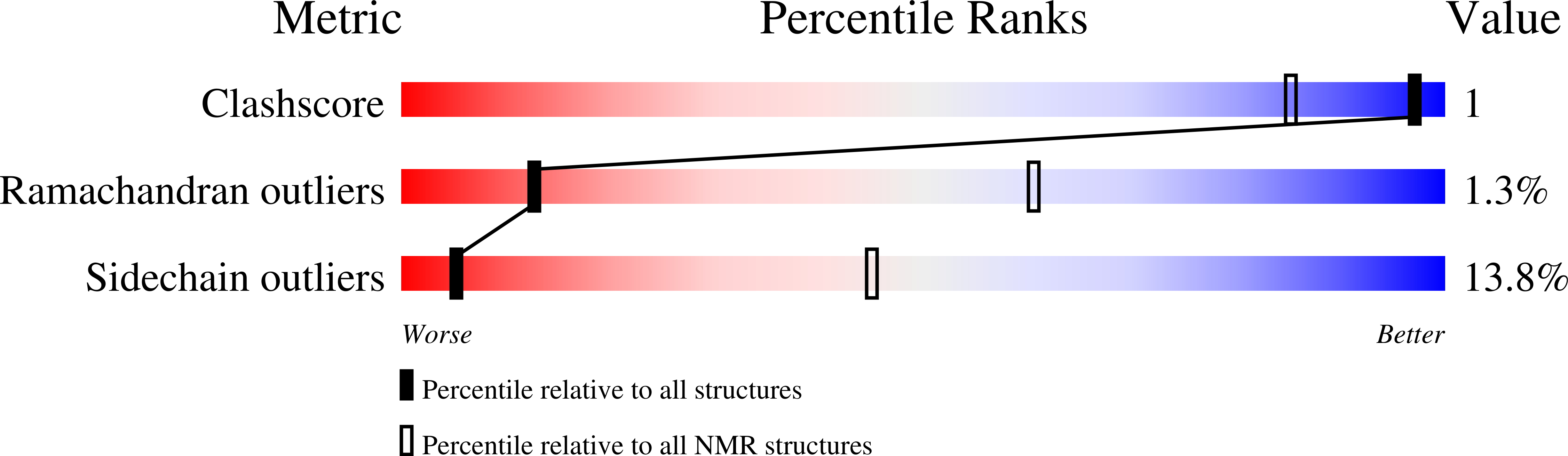Insights into function, catalytic mechanism, and fold evolution of selenoprotein methionine sulfoxide reductase B1 through structural analysis
Aachmann, F.L., Sal, L.S., Kim, H.Y., Marino, S.M., Gladyshev, V.N., Dikiy, A.(2010) J Biological Chem 285: 33315-33323
- PubMed: 20605785
- DOI: https://doi.org/10.1074/jbc.M110.132308
- Primary Citation of Related Structures:
2KV1 - PubMed Abstract:
Methionine sulfoxide reductases protect cells by repairing oxidatively damaged methionine residues in proteins. Here, we report the first three-dimensional structure of the mammalian selenoprotein methionine sulfoxide reductase B1 (MsrB1), determined by high resolution NMR spectroscopy. Heteronuclear multidimensional spectra yielded NMR spectral assignments for the reduced form of MsrB1 in which catalytic selenocysteine (Sec) was replaced with cysteine (Cys). MsrB1 consists of a central structured core of two β-sheets and a highly flexible, disordered N-terminal region. Analysis of pH dependence of NMR signals of catalytically relevant residues, comparison with the data for bacterial MsrBs, and NMR-based structural analysis of methionine sulfoxide (substrate) and methionine sulfone (inhibitor) binding to MsrB1 at the atomic level reveal a mechanism involving catalytic Sec(95) and resolving Cys(4) residues in catalysis. The MsrB1 structure differs from the structures of Cys-containing MsrBs in the use of distal selenenylsulfide, residues needed for catalysis, and the mode in which the active form of the enzyme is regenerated. In addition, this is the first structure of a eukaryotic zinc-containing MsrB, which highlights the structural role of this metal ion bound to four conserved Cys. We integrated this information into a structural model of evolution of MsrB superfamily.
- From the Department of Biotechnology, Norwegian University of Science and Technology, N-7491 Trondheim, Norway.
Organizational Affiliation: