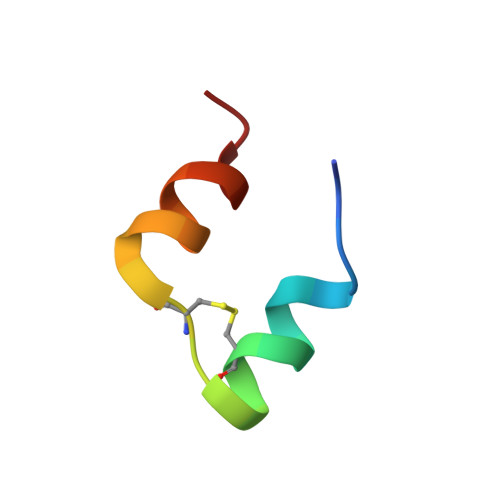
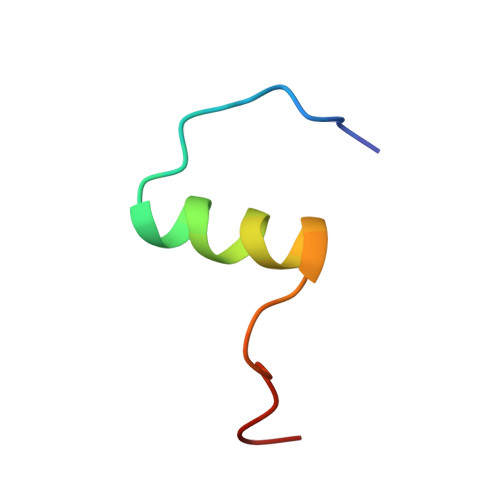
Solution structure of a conformationally restricted fully active derivative of the human relaxin-like factor
Bullesbach, E.E., Hass, M.A.S., Jensen, M.R., Hansen, D.F., Kristensen, S.M., Schwabe, C., Led, J.J.(2008) Biochemistry 47: 13308-13317
- PubMed: 19086273
- DOI: https://doi.org/10.1021/bi801412w
- Primary Citation of Related Structures:
2K6T, 2K6U - PubMed Abstract:
Analogous to insulin, the relaxin-like factor (RLF) must undergo a structural transition to the active form prior to receptor binding. Thus, the C-terminus of the B chain of RLF folds toward the surface of the central B chain helix, causing partial obliteration of the two essential RLF receptor-binding site residues, valine B19 and tryptophan B27. Via comparison of the solution structure of a fully active C-terminally cross-linked RLF analogue with the native synthetic human RLF (hRLF), it became clear that the cross-linked analogue largely retains the essential folding of the native protein. Both proteins exist in a major and minor conformation, as revealed by multiple resonances from tryptophan B27 and adjacent residues on the B chain helix. Notably, the minor conformation is significantly more highly populated in the chemically cross-linked RLF than it is in the hRLF. In addition, compared to the unmodified molecule, subtle differences are observed within the B chain helix whereby the cross-linked derivative shows a reduced level of hydrogen bonding and significant peak broadening at the binding site residue ValB19. On the basis of these observations, we suggest that the solution structure of the native hormone represents an inactive conformer and that a dynamic equilibrium exists between the C-terminally unfolded binding conformation and the inactive conformation of the RLF.
- Department of Biochemistry and Molecular Biology, Medical University of South Carolina, 173 Ashley Avenue, P.O. Box 250509, Charleston, South Carolina 29425, USA. bullesee@musc.edu
Organizational Affiliation: