Identification of a Novel Determinant for Membrane Association in Hepatitis C Virus Nonstructural Protein 4B
Gouttenoire, J., Castet, V., Montserret, R., Arora, N., Raussens, V., Ruysschaert, J.-M., Diesis, E., Blum, H.E., Penin, F., Moradpour, D.(2009) J Virol 83: 6257-6268
- PubMed: 19357161
- DOI: https://doi.org/10.1128/JVI.02663-08
- Primary Citation of Related Structures:
2JXF - PubMed Abstract:
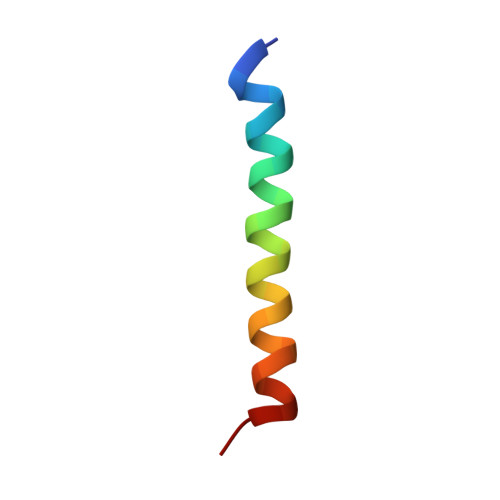
Nonstructural protein 4B (NS4B) plays an essential role in the formation of the hepatitis C virus (HCV) replication complex. It is a relatively poorly characterized integral membrane protein predicted to comprise four transmembrane segments in its central portion. Here, we describe a novel determinant for membrane association represented by amino acids (aa) 40 to 69 in the N-terminal portion of NS4B. This segment was sufficient to target and tightly anchor the green fluorescent protein to cellular membranes, as assessed by fluorescence microscopy as well as membrane extraction and flotation analyses. Circular dichroism and nuclear magnetic resonance structural analyses showed that this segment comprises an amphipathic alpha-helix extending from aa 42 to 66. Attenuated total reflection infrared spectroscopy and glycosylation acceptor site tagging revealed that this amphipathic alpha-helix has the potential to traverse the phospholipid bilayer as a transmembrane segment, likely upon oligomerization. Alanine substitution of the fully conserved aromatic residues on the hydrophobic helix side abrogated membrane association of the segment comprising aa 40 to 69 and disrupted the formation of a functional replication complex. These results provide the first atomic resolution structure of an essential membrane-associated determinant of HCV NS4B.
- Division of Gastroenterology and Hepatology, Centre Hospitalier Universitaire Vaudois, University of Lausanne, Lausanne, Switzerland.
Organizational Affiliation: