Novel Nerve-Agent Antidote Design Based on Crystallographic and Mass Spectrometric Analyses of Tabun-Conjugated Acetylcholinesterase in Complex with Antidotes.
Ekstrom, F.J., Astot, C., Pang, Y.(2007) Clin Pharmacol Ther 82: 282
- PubMed: 17443135
- DOI: https://doi.org/10.1038/sj.clpt.6100151
- Primary Citation of Related Structures:
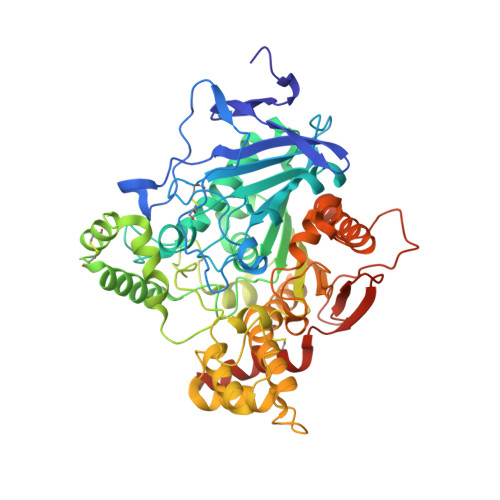
2JEY, 2JEZ, 2JF0 - PubMed Abstract:
Organophosphorus compound-based nerve agents inhibit the essential enzyme acetylcholinesterase (AChE) causing acute toxicity and death. Clinical treatment of nerve-agent poisoning is to use oxime-based antidotes to reactivate the inhibited AChE. However, the nerve agent tabun is resistant to oximes. To design improved oximes, crystal structures of a tabun-conjugated AChE in complex with different oximes are needed to guide the structural modifications of known antidotes. However, this type of structure is extremely challenging to obtain because both deamidation of the tabun conjugate and reactivation of AChE occur during crystallographic experiments. Here we report, for the first time, the crystal structures of Ortho-7 and HLö-7 in complex with AChE that is conjugated to an intact tabun. These structures were determined by our new strategy of combining crystallographic and mass spectrometric analyses of AChE crystals. The results explain the relative reactivation potencies of the two oximes and offer insights into improving known medical antidotes.
- FOI CBRN Defence and Security, Umeå, Sweden. fredrik.ekstrom@foi.se
Organizational Affiliation: