Operator Assisted Harvesting of Protein Crystals Using a Universal Micromanipulation Robot.
Viola, R., Carman, P., Walsh, J., Miller, E., Benning, M., Frankel, D., McPherson, A., Cudney, R., Rupp, B.(2007) J Appl Crystallogr 40: 539
- PubMed: 19461845
- DOI: https://doi.org/10.1107/S0021889807012149
- Primary Citation of Related Structures:
2J9N - PubMed Abstract:
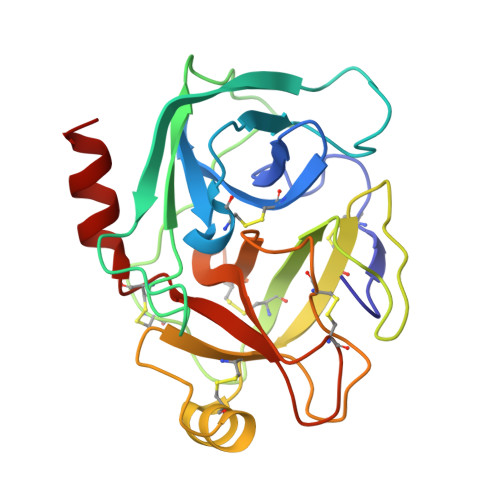
High-throughput crystallography has reached a level of automation where complete computer-assisted robotic crystallization pipelines are capable of cocktail preparation, crystallization plate setup, and inspection and interpretation of results. While mounting of crystal pins, data collection and structure solution are highly automated, crystal harvesting and cryocooling remain formidable challenges towards full automation. To address the final frontier in achieving fully automated high-throughput crystallography, the prototype of an anthropomorphic six-axis universal micromanipulation robot (UMR) has been designed and tested; this UMR is capable of operator-assisted harvesting and cryoquenching of protein crystals as small as 10 microm from a variety of 96-well plates. The UMR is equipped with a versatile tool exchanger providing full operational flexibility. Trypsin crystals harvested and cryoquenched using the UMR have yielded a 1.5 A structure demonstrating the feasibility of robotic protein crystal harvesting.