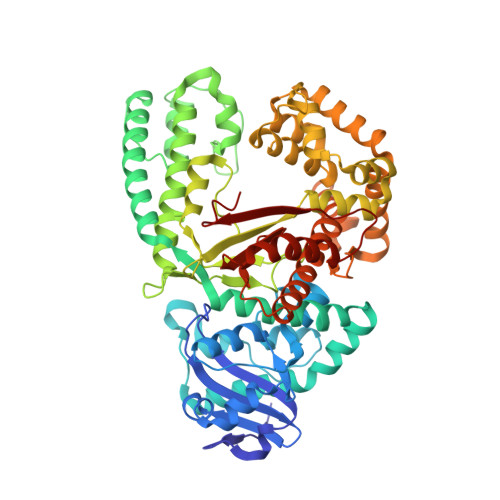
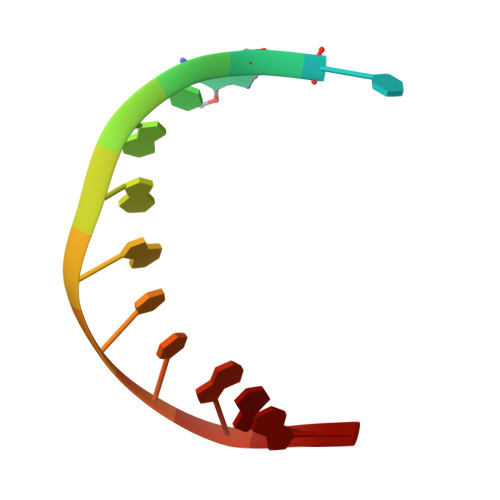
The structural basis for the mutagenicity of O6-methyl-guanine lesions.
Warren, J.J., Forsberg, L.J., Beese, L.S.(2006) Proc Natl Acad Sci U S A 103: 19701-19706
- PubMed: 17179038
- DOI: https://doi.org/10.1073/pnas.0609580103
- Primary Citation of Related Structures:
2HHQ, 2HHS, 2HHT, 2HHU, 2HHV, 2HHW, 2HHX, 2HVH, 2HVI, 2HW3 - PubMed Abstract:
Methylating agents are widespread environmental carcinogens that generate a broad spectrum of DNA damage. Methylation at the guanine O(6) position confers the greatest mutagenic and carcinogenic potential. DNA polymerases insert cytosine and thymine with similar efficiency opposite O(6)-methyl-guanine (O6MeG). We combined pre-steady-state kinetic analysis and a series of nine x-ray crystal structures to contrast the reaction pathways of accurate and mutagenic replication of O6MeG in a high-fidelity DNA polymerase from Bacillus stearothermophilus. Polymerases achieve substrate specificity by selecting for nucleotides with shape and hydrogen-bonding patterns that complement a canonical DNA template. Our structures reveal that both thymine and cytosine O6MeG base pairs evade proofreading by mimicking the essential molecular features of canonical substrates. The steric mimicry depends on stabilization of a rare cytosine tautomer in C.O6MeG-polymerase complexes. An unusual electrostatic interaction between O-methyl protons and a thymine carbonyl oxygen helps stabilize T.O6MeG pairs bound to DNA polymerase. Because DNA methylators constitute an important class of chemotherapeutic agents, the molecular mechanisms of replication of these DNA lesions are important for our understanding of both the genesis and treatment of cancer.
- Department of Biochemistry, Duke University Medical Center, Box 3711, Durham, NC 27710, USA.
Organizational Affiliation: