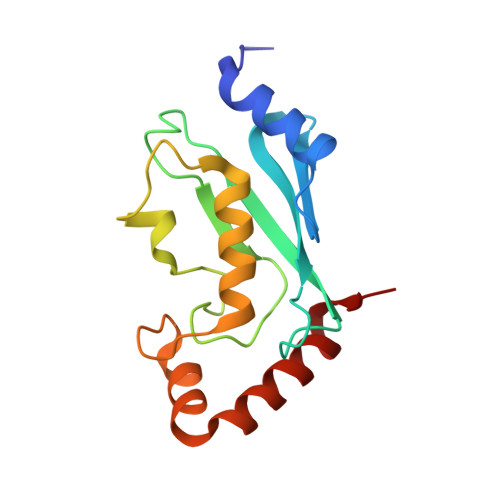
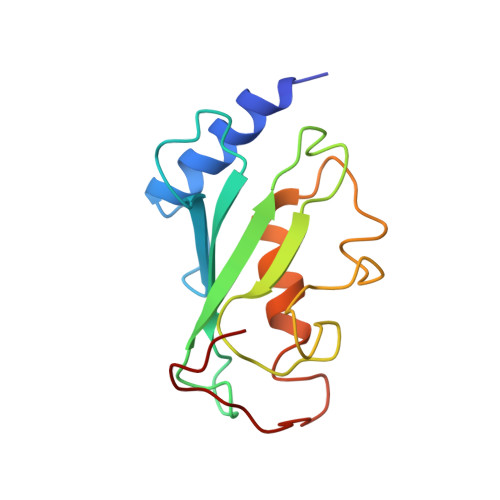
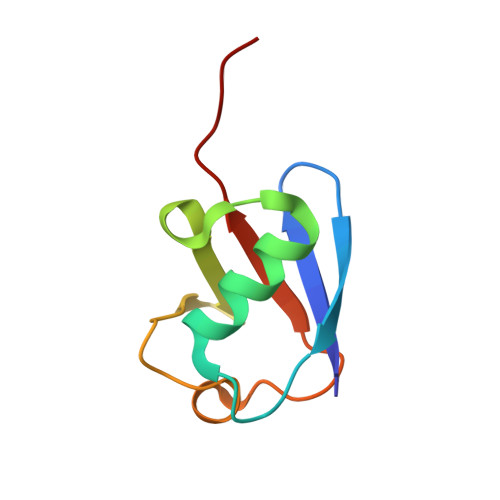
Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation.
Eddins, M.J., Carlile, C.M., Gomez, K.M., Pickart, C.M., Wolberger, C.(2006) Nat Struct Mol Biol 13: 915-920
- PubMed: 16980971
- DOI: https://doi.org/10.1038/nsmb1148
- Primary Citation of Related Structures:
2GMI - PubMed Abstract:
Lys63-linked polyubiquitin chains participate in nonproteolytic signaling pathways, including regulation of DNA damage tolerance and NF-kappaB activation. E2 enzymes bound to ubiquitin E2 variants (UEV) are vital in these pathways, synthesizing Lys63-linked polyubiquitin chains, but how these complexes achieve specificity for a particular lysine linkage has been unclear. We have determined the crystal structure of an Mms2-Ubc13-ubiquitin (UEV-E2-Ub) covalent intermediate with donor ubiquitin linked to the active site residue of Ubc13. In the structure, the unexpected binding of a donor ubiquitin of one Mms2-Ubc13-Ub complex to the acceptor-binding site of Mms2-Ubc13 in an adjacent complex allows us to visualize at atomic resolution the molecular determinants of acceptor-ubiquitin binding. The structure reveals the key role of Mms2 in allowing selective insertion of Lys63 into the Ubc13 active site and suggests a molecular model for polyubiquitin chain elongation.
- Department of Biophysics and Biophysical Chemistry and the Howard Hughes Medical Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, USA.
Organizational Affiliation: