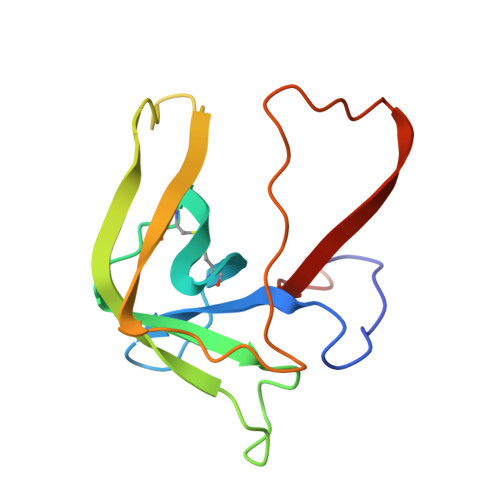
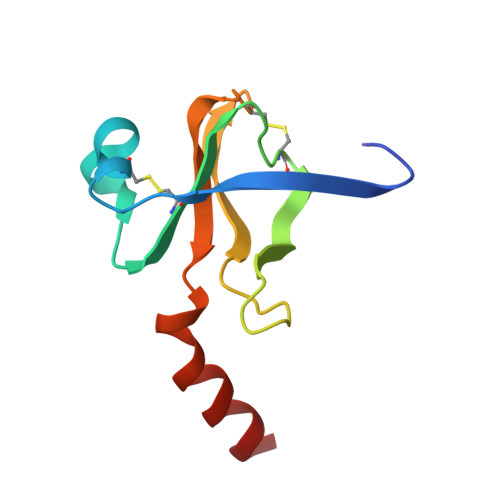
Refined crystal structure of gamma-chymotrypsin at 1.9 A resolution. Comparison with other pancreatic serine proteases.
Cohen, G.H., Silverton, E.W., Davies, D.R.(1981) J Mol Biology 148: 449-479
- PubMed: 6914398
- DOI: https://doi.org/10.1016/0022-2836(81)90186-8
- Primary Citation of Related Structures:
2GCH