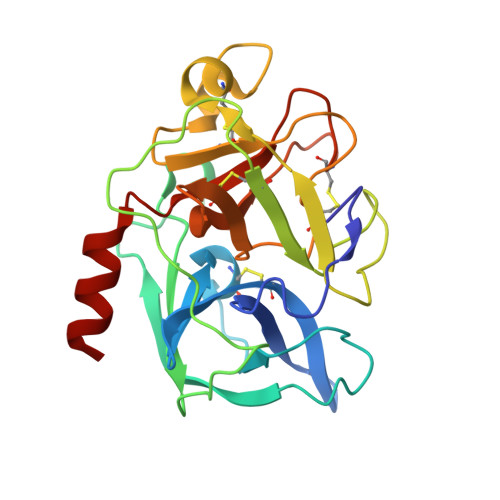
Structure of the complex of porcine pancreatic elastase with a trimacrocyclic peptide inhibitor FR901451
Kinoshita, T., Kitatani, T., Warizaya, M., Tada, T.(2005) Acta Crystallogr Sect F Struct Biol Cryst Commun 61: 808-811
- PubMed: 16511165
- DOI: https://doi.org/10.1107/S1744309105026047
- Primary Citation of Related Structures:
2CV3 - PubMed Abstract:
Porcine pancreatic elastase (PPE) resembles the attractive drug target leukocyte elastase, which has the ability to degrade connective tissue in the body. The crystal structure of PPE complexed with a novel trimacrocyclic peptide inhibitor, FR901451, was solved at 1.9 A resolution. The inhibitor occupied the subsites S3 through S3' of PPE and induced conformational changes in the side chains of Arg64 and Arg226, which are located at the edges of the substrate-binding cleft. Structural comparison of five PPE-inhibitor complexes, including the FR901451 complex and non-ligated PPE, reveals that the residues forming the S2, S1, S1' and S2' subsites in the cleft are rigid, but the two arginine residues playing a part in the S3 and S3' subsites are flexible. Structural comparison of PPE with human leukocyte elastase (HLE) implies that the inhibitor binds to HLE in a similar manner to the FR901451-PPE complex. This structural insight may help in the design of potent elastase inhibitors.
- Department of Biological Science, Graduate School of Science, Osaka Prefecture University, Sakai, Osaka 599-8531, Japan.
Organizational Affiliation: