Ofloxacin-Like Antibiotics Inhibit Pneumococcal Cell Wall-Degrading Virulence Factors
Fernandez-Tornero, C., Garcia, E., Lopez, R., Pascual-Teresa, B.D., Gimenez-Gallego, G., Romero, A.(2005) J Biological Chem 280: 19948
- PubMed: 15769740
- DOI: https://doi.org/10.1074/jbc.M501236200
- Primary Citation of Related Structures:
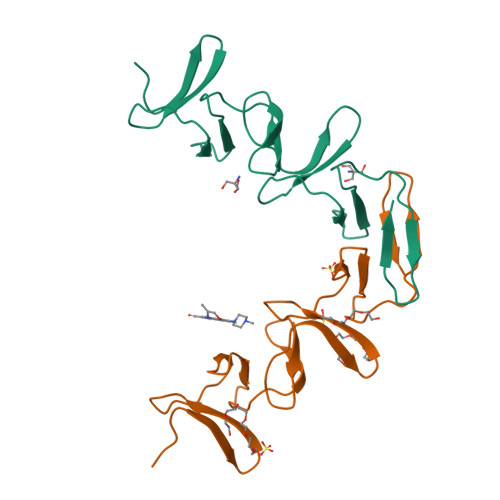
2BML - PubMed Abstract:
The search for new drugs against Streptococcus pneumoniae (pneumococcus) is driven by the 1.5 million deaths it causes annually. Choline-binding proteins attach to the pneumococcal cell wall through domains that recognize choline moieties, and their involvement in pneumococcal virulence makes them potential targets for drug development. We have defined chemical criteria involved in the docking of small molecules from a three-dimensional structural library to the major pneumococcal autolysin (LytA) choline binding domain. These criteria were used to identify compounds that could interfere with the attachment of this protein to the cell wall, and several quinolones that fit this framework were found to inhibit the cell wall-degrading activity of LytA. Furthermore, these compounds produced similar effects on other enzymes with different catalytic activities but that contained a similar choline binding domain; that is, autolysin (LytC) and the phage lytic enzyme (Cpl-1). Finally, we resolved the crystal structure of the complex between the choline binding domain of LytA and ofloxacin at a resolution of 2.6 Angstroms. These data constitute an important launch pad from which effective drugs to combat pneumococcal infections can be developed.
Organizational Affiliation:
Departamento de Estructura y Función de Proteínas, Consejo Superior de Investigaciones Científicas, Madrid, Spain.