Inaugural Article: On the mechanism of sensing unfolded protein in the endoplasmic reticulum
Credle, J.J., Finer-Moore, J.S., Papa, F.R., Stroud, R.M., Walter, P.(2005) Proc Natl Acad Sci U S A 102: 18773-18784
- PubMed: 16365312
- DOI: https://doi.org/10.1073/pnas.0509487102
- Primary Citation of Related Structures:
2BE1 - PubMed Abstract:
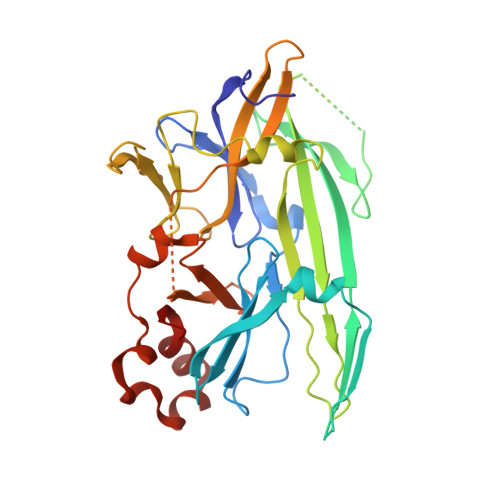
Unfolded proteins in the endoplasmic reticulum (ER) activate the ER transmembrane sensor Ire1 to trigger the unfolded protein response (UPR), a homeostatic signaling pathway that adjusts ER protein folding capacity according to need. Ire1 is a bifunctional enzyme, containing cytoplasmic kinase and RNase domains whose roles in signal transduction downstream of Ire1 are understood in some detail. By contrast, the question of how its ER-luminal domain (LD) senses unfolded proteins has remained an enigma. The 3.0-A crystal structure and consequent structure-guided functional analyses of the conserved core region of the LD (cLD) leads us to a proposal for the mechanism of response. cLD exhibits a unique protein fold and is sufficient to control Ire1 activation by unfolded proteins. Dimerization of cLD monomers across a large interface creates a shared central groove formed by alpha-helices that are situated on a beta-sheet floor. This groove is reminiscent of the peptide binding domains of major histocompatibility complexes (MHCs) in its gross architecture. Conserved amino acid side chains in Ire1 that face into the groove are shown to be important for UPR activation in that their mutation reduces the response. Mutational analyses suggest that further interaction between cLD dimers is required to form higher-order oligomers necessary for UPR activation. We propose that cLD directly binds unfolded proteins, which changes the quaternary association of the monomers in the membrane plane. The changes in the ER lumen in turn position Ire1 kinase domains in the cytoplasm optimally for autophosphorylation to initiate the UPR.
- Howard Hughes Medical Institute, Departments of Biochemistry and Biophysics and Medicine, University of California, San Francisco, CA 94143-2200, USA.
Organizational Affiliation: