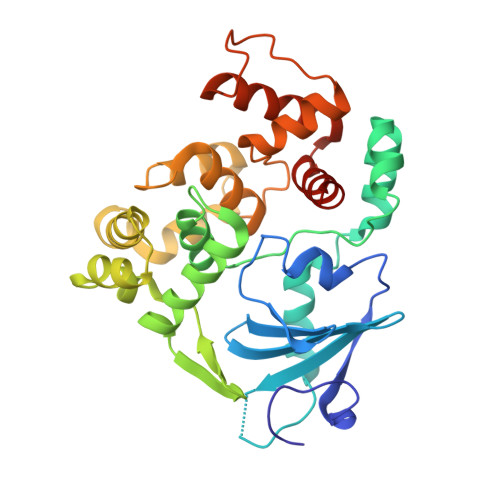
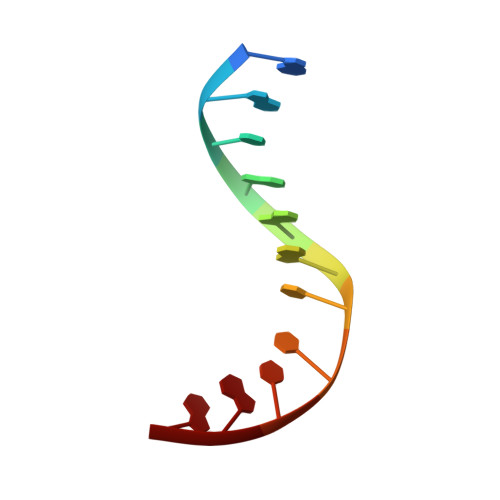
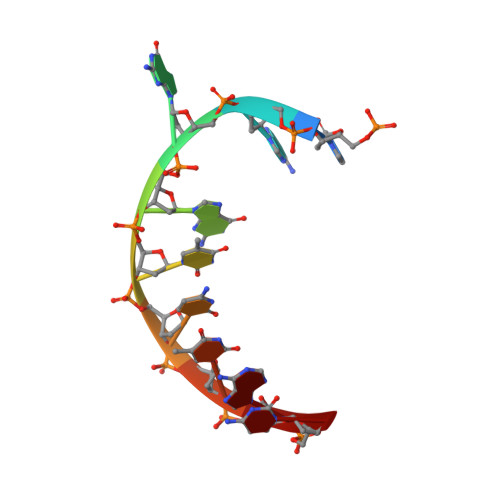Structure of a repair enzyme interrogating undamaged DNA elucidates recognition of damaged DNA.
Banerjee, A., Yang, W., Karplus, M., Verdine, G.L.(2005) Nature 434: 612-618
- PubMed: 15800616
- DOI: https://doi.org/10.1038/nature03458
- Primary Citation of Related Structures:
1YQK, 1YQL, 1YQM, 1YQR - PubMed Abstract:
How DNA repair proteins distinguish between the rare sites of damage and the vast expanse of normal DNA is poorly understood. Recognizing the mutagenic lesion 8-oxoguanine (oxoG) represents an especially formidable challenge, because this oxidized nucleobase differs by only two atoms from its normal counterpart, guanine (G). Here we report the use of a covalent trapping strategy to capture a human oxoG repair protein, 8-oxoguanine DNA glycosylase I (hOGG1), in the act of interrogating normal DNA. The X-ray structure of the trapped complex features a target G nucleobase extruded from the DNA helix but denied insertion into the lesion recognition pocket of the enzyme. Free energy difference calculations show that both attractive and repulsive interactions have an important role in the preferential binding of oxoG compared with G to the active site. The structure reveals a remarkably effective gate-keeping strategy for lesion discrimination and suggests a mechanism for oxoG insertion into the hOGG1 active site.
- Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, Massachusetts 02138, USA.
Organizational Affiliation: