A crystal structure of IAA acetyltransferase from Bacillus cereus
Nocek, B.P., Osipiuk, J., Li, H., Collart, F., Joachimiak, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| IAA acetyltransferase | 157 | Bacillus cereus ATCC 14579 | Mutation(s): 7 EC: 2.3.1 | 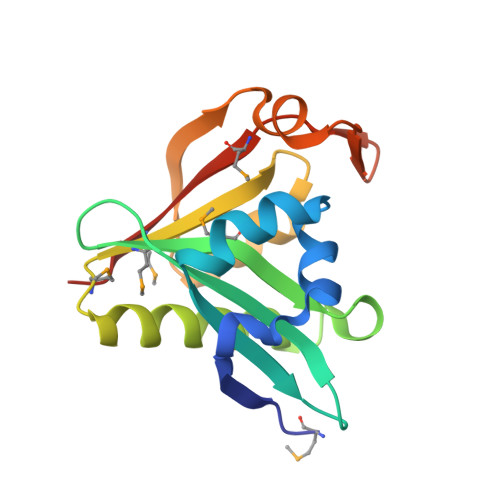 | |
UniProt | |||||
Find proteins for Q81FK8 (Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG 7414 / JCM 2152 / NBRC 15305 / NCIMB 9373 / NCTC 2599 / NRRL B-3711)) Explore Q81FK8 Go to UniProtKB: Q81FK8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q81FK8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 70.122 | α = 90 |
| b = 70.122 | β = 90 |
| c = 171.516 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| HKL-3000 | phasing |
| MAID | phasing |