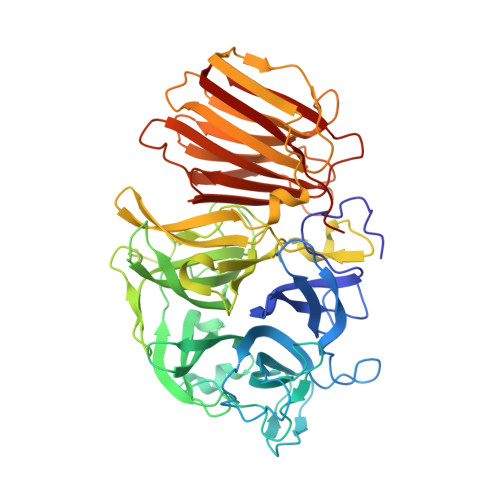
Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition
Nagem, R.A.P., Rojas, A.L., Golubev, A.M., Korneeva, O.S., Eneyskaya, E.V., Kulminskaya, A.A., Neustroev, K.N., Polikarpov, I.(2004) J Mol Biology 344: 471-480
- PubMed: 15522299
- DOI: https://doi.org/10.1016/j.jmb.2004.09.024
- Primary Citation of Related Structures:
1Y4W, 1Y9G, 1Y9M - PubMed Abstract:
Exo-inulinases hydrolyze terminal, non-reducing 2,1-linked and 2,6-linked beta-d-fructofuranose residues in inulin, levan and sucrose releasing beta-d-fructose. We present the X-ray structure at 1.55A resolution of exo-inulinase from Aspergillus awamori, a member of glycoside hydrolase family 32, solved by single isomorphous replacement with the anomalous scattering method using the heavy-atom sites derived from a quick cryo-soaking technique. The tertiary structure of this enzyme folds into two domains: the N-terminal catalytic domain of an unusual five-bladed beta-propeller fold and the C-terminal domain folded into a beta-sandwich-like structure. Its structural architecture is very similar to that of another member of glycoside hydrolase family 32, invertase (beta-fructosidase) from Thermotoga maritima, determined recently by X-ray crystallography The exo-inulinase is a glycoprotein containing five N-linked oligosaccharides. Two crystal forms obtained under similar crystallization conditions differ by the degree of protein glycosylation. The X-ray structure of the enzyme:fructose complex, at a resolution of 1.87A, reveals two catalytically important residues: Asp41 and Glu241, a nucleophile and a catalytic acid/base, respectively. The distance between the side-chains of these residues is consistent with a double displacement mechanism of reaction. Asp189, which is part of the Arg-Asp-Pro motif, provides hydrogen bonds important for substrate recognition.
- Instituto de Física de São Carlos, Universidade de São Paulo, Av. Trabalhador São-carlense 400, CEP 13560-970, São Carlos, SP, Brazil.
Organizational Affiliation: