Structure and dynamics of micelle-bound human alpha-synuclein
Ulmer, T.S., Bax, A., Cole, N.B., Nussbaum, R.L.(2005) J Biological Chem 280: 9595-9603
- PubMed: 15615727
- DOI: https://doi.org/10.1074/jbc.M411805200
- Primary Citation of Related Structures:
1XQ8 - PubMed Abstract:
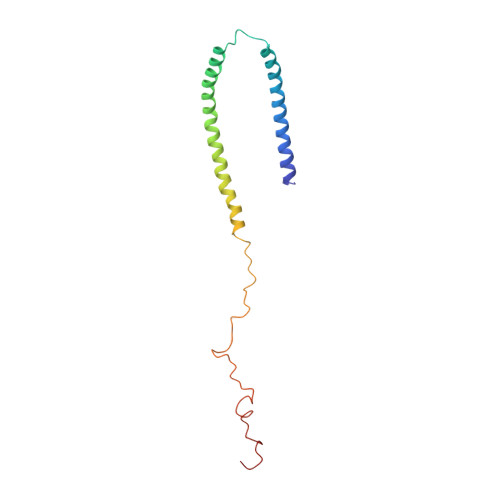
Misfolding of the protein alpha-synuclein (aS), which associates with presynaptic vesicles, has been implicated in the molecular chain of events leading to Parkinson's disease. Here, the structure and dynamics of micelle-bound aS are reported. Val3-Val37 and Lys45-Thr92 form curved alpha-helices, connected by a well ordered, extended linker in an unexpected anti-parallel arrangement, followed by another short extended region (Gly93-Lys97), overlapping the recently identified chaperone-mediated autophagy recognition motif and a highly mobile tail (Asp98-Ala140). Helix curvature is significantly less than predicted based on the native micelle shape, indicating a deformation of the micelle by aS. Structural and dynamic parameters show a reduced helical content for Ala30-Val37. A dynamic variation in interhelical distance on the microsecond timescale is complemented by enhanced sub-nanosecond timescale dynamics, particularly in the remarkably glycine-rich segments of the helices. These unusually rich dynamics may serve to mitigate the effect of aS binding on membrane fluidity. The well ordered conformation of the helix-helix connector indicates a defined interaction with lipidic surfaces, suggesting that, when bound to larger diameter synaptic vesicles, it can act as a switch between this structure and a previously proposed uninterrupted helix.
- Laboratory of Chemical Physics, NIDDK, National Institutes of Health, Bethesda, Maryland 20892 , USA. tobias.ulmer@nih.gov
Organizational Affiliation: