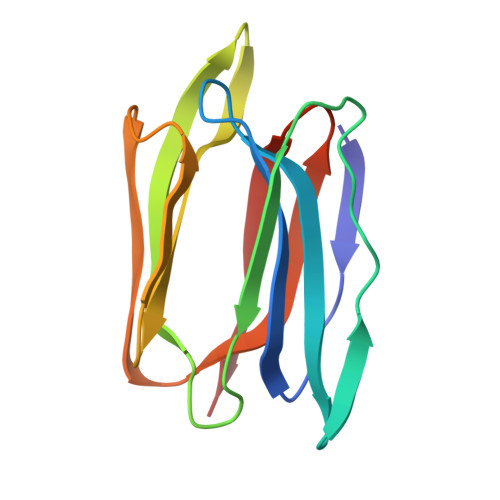
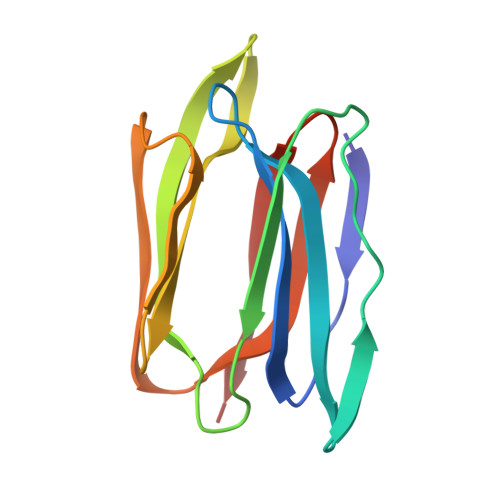
Structural basis for the energetics of jacalin-sugar interactions: promiscuity versus specificity
Jeyaprakash, A.A., Jayashree, G., Mahanta, S.K., Swaminathan, C.P., Sekar, K., Surolia, A., Vijayan, M.(2005) J Mol Biology 347: 181-188
- PubMed: 15733927
- DOI: https://doi.org/10.1016/j.jmb.2005.01.015
- Primary Citation of Related Structures:
1WS4, 1WS5 - PubMed Abstract:
Jacalin, a tetrameric lectin, is one of the two lectins present in jackfruit (Artocarpus integrifolia) seeds. Its crystal structure revealed, for the first time, the occurrence of the beta-prism I fold in lectins. The structure led to the elucidation of the crucial role of a new N terminus generated by post-translational proteolysis for the lectin's specificity for galactose. Subsequent X-ray studies on other carbohydrate complexes showed that the extended binding site of jacalin consisted of, in addition to the primary binding site, a hydrophobic secondary site A composed of aromatic residues and a secondary site B involved mainly in water-bridges. A recent investigation involving surface plasmon resonance and the X-ray analysis of a methyl-alpha-mannose complex, had led to a suggestion of promiscuity in the lectin's sugar specificity. To explore this suggestion further, detailed isothermal titration calorimetric studies on the interaction of galactose (Gal), mannose (Man), glucose (Glc), Me-alpha-Gal, Me-alpha-Man, Me-alpha-Glc and other mono- and oligosaccharides of biological relevance and crystallographic studies on the jacalin-Me-alpha-Glc complex and a new form of the jacalin-Me-alpha-Man complex, have been carried out. The binding affinity of Me-alpha-Man is 20 times weaker than that of Me-alpha-Gal. The corresponding number is 27, when the binding affinities of Gal and Me-alpha-Gal, and those of Man and Me-alpha-Man are compared. Glucose (Glc) shows no measurable binding, while the binding affinity of Me-alpha-Glc is slightly less than that of Me-alpha-Man. The available crystal structures of jacalin-sugar complexes provide a convincing explanation for the energetics of binding in terms of interactions at the primary binding site and secondary site A. The other sugars used in calorimetric studies show no detectable binding to jacalin. These results and other available evidence suggest that jacalin is specific to O-glycans and its affinity to N-glycans is extremely weak or non-existent and therefore of limited value in processes involving biological recognition.
- Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India.
Organizational Affiliation: