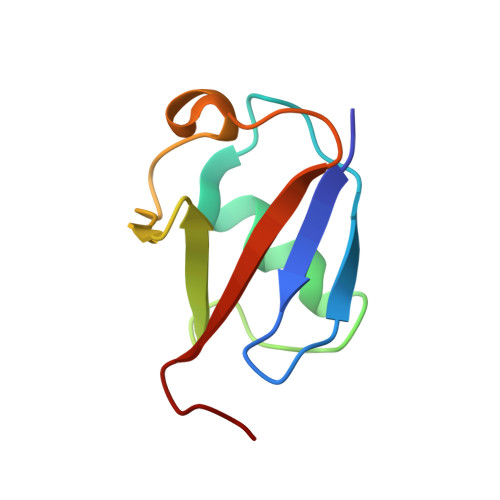
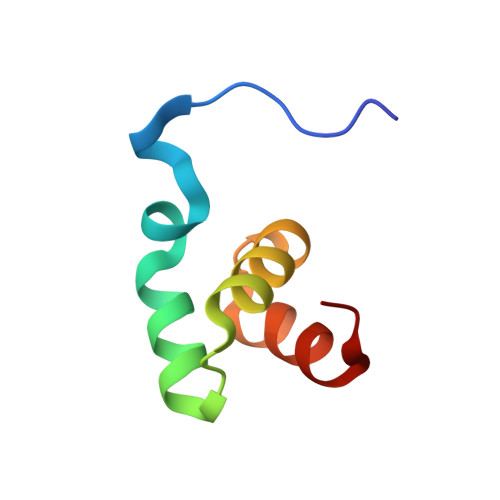
Structure of the UBA domain of Dsk2p in complex with ubiquitin molecular determinants for ubiquitin recognition.
Ohno, A., Jee, J., Fujiwara, K., Tenno, T., Goda, N., Tochio, H., Kobayashi, H., Hiroaki, H., Shirakawa, M.(2005) Structure 13: 521-532
- PubMed: 15837191
- DOI: https://doi.org/10.1016/j.str.2005.01.011
- Primary Citation of Related Structures:
1WR1 - PubMed Abstract:
The ubiquitin-associated (UBA) domain is one of the most frequently occurring motifs that recognize ubiquitin tags. Dsk2p, a UBA-containing protein from Saccharomyces cerevisiae, is involved in the ubiquitin-proteasome proteolytic pathway and has been implicated in spindle pole duplication. Here we present the solution structure of the UBA domain of Dsk2p (Dsk2(UBA)) in complex with ubiquitin. The structure reveals that the UBA domain uses a mode of ubiquitin recognition that is similar to that of the CUE domain, another ubiquitin binding motif that shares low sequence homology but high structural similarity with UBA domains. These two domains, as well as the structurally unrelated ubiquitin binding motif UIM, provide a common, crucial recognition site for ubiquitin, comprising a hydrogen-bonding acceptor for the amide group of Gly-47, and a methyl group that packs against the hydrophobic pocket of ubiquitin formed by Leu-8, Ile-44, His-68, and Val-70.
- Graduate School of Integrated Science, Yokohama City University, Yokohama, Kanagawa 230-0045, Japan.
Organizational Affiliation: