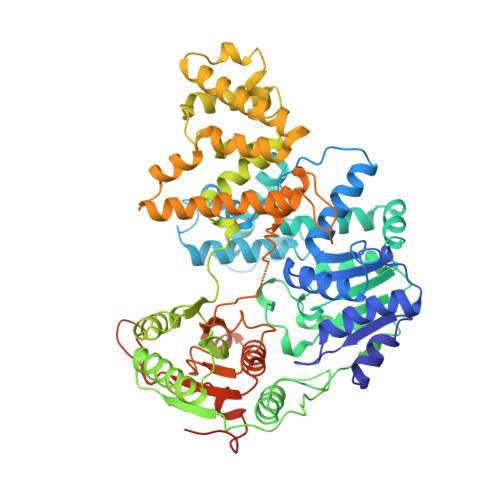
Major domain swiveling revealed by the crystal structures of complexes of E. coli Rep helicase bound to single-stranded DNA and ADP.
Korolev, S., Hsieh, J., Gauss, G.H., Lohman, T.M., Waksman, G.(1997) Cell 90: 635-647
- PubMed: 9288744
- DOI: https://doi.org/10.1016/s0092-8674(00)80525-5
- Primary Citation of Related Structures:
1UAA - PubMed Abstract:
Crystal structures of binary and ternary complexes of the E. coli Rep helicase bound to single-stranded (ss) DNA or ssDNA and ADP were determined to a resolution of 3.0 A and 3.2 A, respectively. The asymmetric unit in the crystals contains two Rep monomers differing from each other by a large reorientation of one of the domains, corresponding to a swiveling of 130 degrees about a hinge region. Such domain movements are sufficiently large to suggest that these may be coupled to translocation of the Rep dimer along DNA. The ssDNA binding site involves the helicase motifs Ia, III, and V, whereas the ADP binding site involves helicase motifs I and IV. Residues in motifs II and VI may function to transduce the allosteric effects of nucleotides on DNA binding. These structures represent the first view of a DNA helicase bound to DNA.
- Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, Missouri 63110, USA.
Organizational Affiliation: