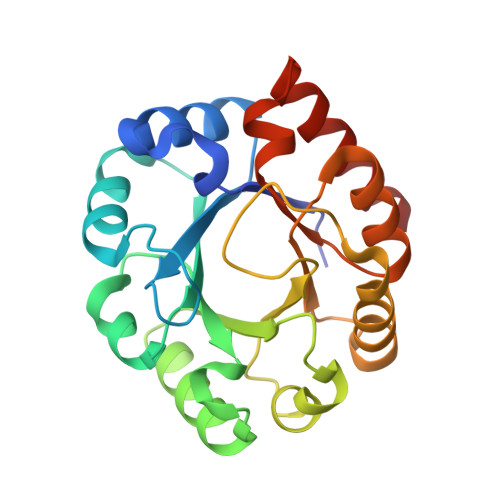
Structure of a ribulose 5-phosphate 3-epimerase from Plasmodium falciparum.
Caruthers, J., Bosch, J., Buckner, F., Van Voorhis, W., Myler, P., Worthey, E., Mehlin, C., Boni, E., DeTitta, G., Luft, J., Lauricella, A., Kalyuzhniy, O., Anderson, L., Zucker, F., Soltis, M., Hol, W.G.(2006) Proteins 62: 338-342
- PubMed: 16304640
- DOI: https://doi.org/10.1002/prot.20764
- Primary Citation of Related Structures:
1TQX - PubMed Abstract:
The crystal structure of Pfal009167AAA, a putative ribulose 5-phosphate 3-epimerase (PfalRPE) from Plasmodium falciparum, has been determined to 2 A resolution. RPE represents an exciting potential drug target for developing antimalarials because it is involved in the shikimate and the pentose phosphate pathways. The structure is a classic TIM-barrel fold. A coordinated Zn ion and a bound sulfate ion in the active site of the enzyme allow for a greater understanding of the mechanism of action of this enzyme. This structure is solved in the framework of the Structural Genomics of Pathogenic Protozoa (SGPP) consortium.
- SLAC, Stanford University, Menlo Park, California, USA.
Organizational Affiliation: