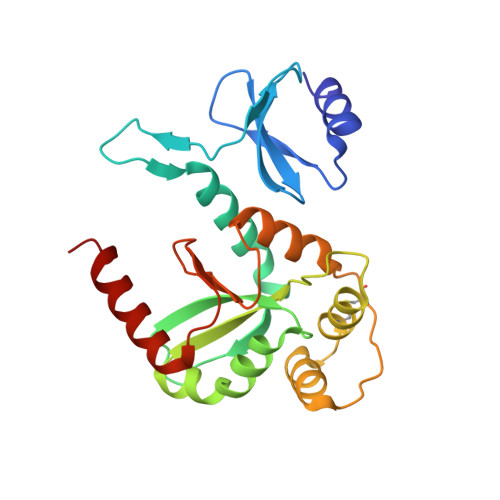
Structure of the reduced disulfide-bond isomerase DsbC from Escherichia coli.
Banaszak, K., Mechin, I., Frost, G., Rypniewski, W.(2004) Acta Crystallogr D Biol Crystallogr 60: 1747-1752
- PubMed: 15388920
- DOI: https://doi.org/10.1107/S0907444904018359
- Primary Citation of Related Structures:
1TJD - PubMed Abstract:
Disufide-bond isomerase (DsbC) plays a crucial role in folding periplasmically excreted bacterial proteins. The crystal structure of the reduced form of DsbC is presented. The pair of thiol groups from Cys98 and Cys101 that form the reversible disulfide bond in the enzymatic active site are 3.1 A apart and the electron density clearly shows that the S atoms do not form a covalent bond. The other pair of Cys residues (141 and 163) in DsbC form a disulfide bond. This is different from the previously reported crystal form of DsbC (McCarthy et al., 2000), in which both Cys pairs are oxidized. Specific hydrogen-bond interactions are identified that stabilize the active site in the reactive reduced state with the special participation of hydrogen bonds between the active-site cysteine residues (98 and 101) and threonine residues 94 and 182. The present structure also differs in the orientation of the catalytic domains within the protein dimer. This is evidence of flexibility within the protein that probably plays a role in accommodating the substrates in the cleft between the catalytic domains.
- Institute of Bioorganic Chemistry, Polish Academy of Sciences, Noskowskiego 12/14, 61-704 Poznan, Poland.
Organizational Affiliation: