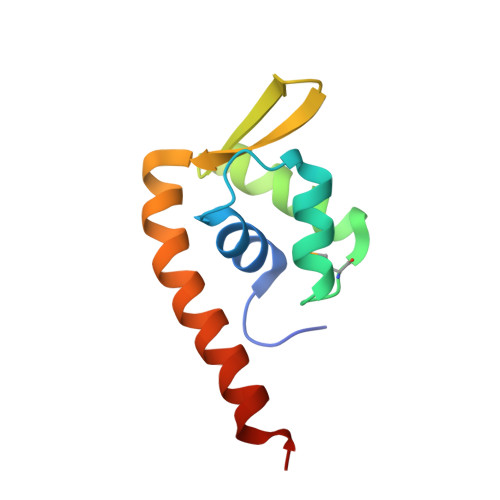
Crystal structure of F-93 from Sulfolobus spindle-shaped virus 1, a winged-helix DNA binding protein.
Kraft, P., Oeckinghaus, A., Kummel, D., Gauss, G.H., Gilmore, J., Wiedenheft, B., Young, M., Lawrence, C.M.(2004) J Virol 78: 11544-11550
- PubMed: 15479795
- DOI: https://doi.org/10.1128/JVI.78.21.11544-11550.2004
- Primary Citation of Related Structures:
1TBX - PubMed Abstract:
Sulfolobus spindle-shaped viruses (SSVs), or Fuselloviridae, are ubiquitous crenarchaeal viruses found in high-temperature acidic hot springs around the world (pH /=70 degrees C). Because they are relatively easy to isolate, they represent the best studied of the crenarchaeal viruses. This is particularly true for the type virus, SSV1, which contains a double-stranded DNA genome of 15.5 kilobases, encoding 34 putative open reading frames. Interestingly, the genome shows little sequence similarity to organisms other than its SSV homologues. Together, sequence similarity and biochemical analyses have suggested functions for only 6 of the 34 open reading frames. Thus, even though SSV1 is the best-studied crenarchaeal virus, functions for most (28) of its open reading frames remain unknown. We have undertaken biochemical and structural studies for the gene product of open reading frame F-93. We find that F-93 exists as a homodimer in solution and that a tight dimer is also present in the 2.7-A crystal structure. Further, the crystal structure reveals a fold that is homologous to the SlyA and MarR subfamilies of winged-helix DNA binding proteins. This strongly suggests that F-93 functions as a transcription factor that recognizes a (pseudo-)palindromic DNA target sequence.
- Thermal Biology Institute, Montana State University, Bozeman 59717, USA.
Organizational Affiliation: