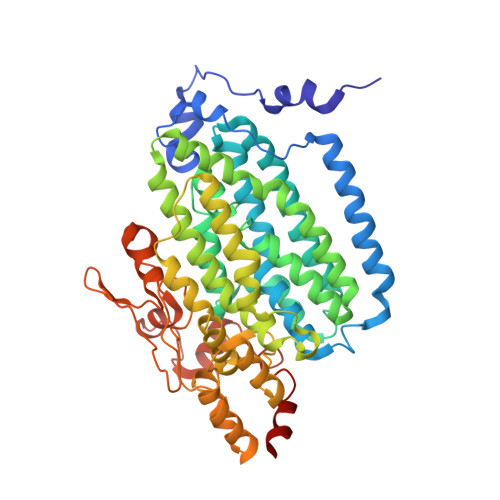
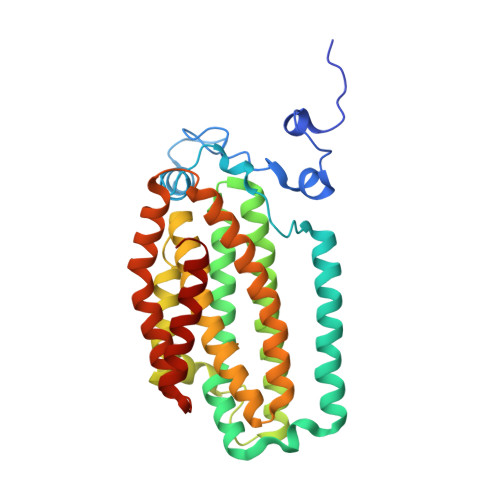
Crystal Structure of the Toluene/o-Xylene Monooxygenase Hydroxylase from Pseudomonas stutzeri OX1: INSIGHT INTO THE SUBSTRATE SPECIFICITY, SUBSTRATE CHANNELING, AND ACTIVE SITE TUNING OF MULTICOMPONENT MONOOXYGENASES.
Sazinsky, M.H., Bard, J., Di Donato, A., Lippard, S.J.(2004) J Biological Chem 279: 30600-30610
- PubMed: 15096510
- DOI: https://doi.org/10.1074/jbc.M400710200
- Primary Citation of Related Structures:
1T0Q, 1T0R, 1T0S - PubMed Abstract:
The four-component toluene/o-xylene monooxygenase (ToMO) from Pseudomonas stutzeri OX1 is capable of oxidizing arenes, alkenes, and haloalkanes at a carboxylate-bridged diiron center similar to that of soluble methane monooxygenase (sMMO). The remarkable variety of substrates accommodated by ToMO invites applications ranging from bioremediation to the regio- and enantiospecific oxidation of hydrocarbons on an industrial scale. We report here the crystal structures of the ToMO hydroxylase (ToMOH), azido ToMOH, and ToMOH containing the product analogue 4-bromophenol to 2.3 A or greater resolution. The catalytic diiron(III) core resembles that of the sMMO hydroxylase, but aspects of the alpha2beta2gamma2 tertiary structure are notably different. Of particular interest is a 6-10 A-wide channel of approximately 35 A in length extending from the active site to the protein surface. The presence of three bromophenol molecules in this space confirms this route as a pathway for substrate entrance and product egress. An analysis of the ToMOH active site cavity offers insights into the different substrate specificities of multicomponent monooxygenases and explains the behavior of mutant forms of homologous enzymes described in the literature.
- Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA.
Organizational Affiliation: