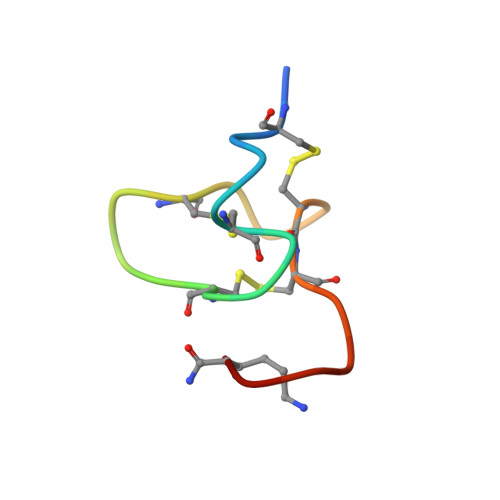
The structure of the Cys-rich terminal domain of Hydra minicollagen, which is involved in disulfide networks of the nematocyst wall.
Pokidysheva, E., Milbradt, A.G., Meier, S., Renner, C., Haussinger, D., Bachinger, H.P., Moroder, L., Grzesiek, S., Holstein, T.W., Ozbek, S., Engel, J.(2004) J Biological Chem 279: 30395-30401
- PubMed: 15123641
- DOI: https://doi.org/10.1074/jbc.M403734200
- Primary Citation of Related Structures:
1SOP - PubMed Abstract:
The minicollagens found in the nematocysts of Hydra constitute a family of invertebrate collagens with unusual properties. They share a common modular architecture with a central collagen sequence ranging from 14 to 16 Gly-X-Y repeats flanked by polyproline/hydroxyproline stretches and short terminal domains that show a conserved cysteine pattern (CXXXCXXXCXXX-CXXXCC). The minicollagen cysteine-rich domains are believed to function in a switch of the disulfide connectivity from intra- to intermolecular bonds during maturation of the capsule wall. The solution structure of the C-terminal fragment including a minicollagen cysteine-rich domain of minicollagen-1 was determined in two independent groups by 1H NMR. The corresponding peptide comprising the last 24 residues of the molecule was produced synthetically and refolded by oxidation under low protein concentrations. Both presented structures are identical in their fold and disulfide connections (Cys2-Cys18, Cys6-Cys14, and Cys10-Cys19) revealing a robust structural motif that is supposed to serve as the polymerization module of the nematocyst capsule.
- Department of Biophysical Chemistry, Biozentrum, University of Basel, Klingelbergstrasse 70, CH-4056 Basel, Switzerland.
Organizational Affiliation: