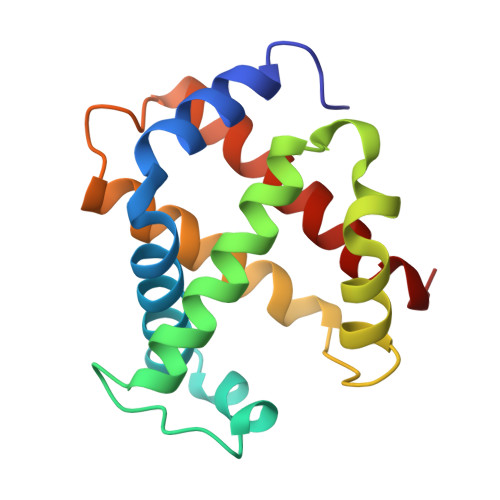
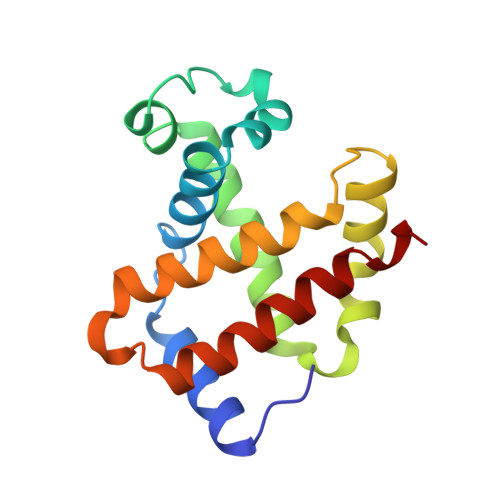
Allosteric transition intermediates modelled by crosslinked haemoglobins.
Schumacher, M.A., Dixon, M.M., Kluger, R., Jones, R.T., Brennan, R.G.(1995) Nature 375: 84-87
- PubMed: 7723849
- DOI: https://doi.org/10.1038/375084a0
- Primary Citation of Related Structures:
1SDK, 1SDL - PubMed Abstract:
The structural end-points of haemoglobin's transition from its low-oxygen-affinity (T) to high-oxygen-affinity (R) state, have been well established by X-ray crystallography, but short-lived intermediates have proved less amenable to X-ray studies. Here we use chemical crosslinking to fix these intermediates for structural characterization. We describe the X-ray structures of three haemoglobins, alpha 2 beta 1S82 beta, alpha 2 beta 1Tm82 beta and alpha 2 beta 1,82Tm82 beta, which were crosslinked between the amino groups of residues beta Val1 and beta Lys82 by 3,3'-stilbenedicarboxylic acid (S) or trimesic acid (Tm) while in the deoxy state, and saturated with carbon monoxide before crystallization. alpha 2 beta 1S82 beta, which has almost normal oxygen affinity, is completely in the R-state conformation; however, alpha 2 beta 1Tm82 beta and alpha 2 beta 1,82Tm82 beta, both of which have low oxygen affinity, have been prevented from completing their transition into the R state and display many features of a transitional intermediate. These haemoglobins therefore represent a snapshot of the nascent R state.
- Department of Biochemistry and Molecular Biology, Oregon Health Sciences University, Portland 97201-3098, USA.
Organizational Affiliation: