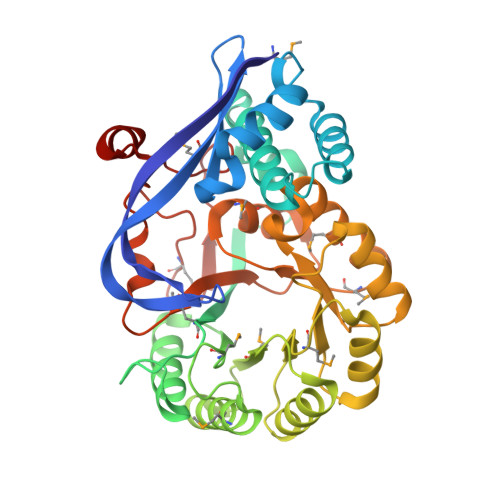
Evolution of enzymatic activites in the Enolase superfamily: 1.7 A crystal structure of the hypothetical protein MR.GI-17937161 from Agrobacterium tumefaciens
Fedorov, A.A., Fedorov, E.V., Thirumuruhan, R., Zencheck, W., Millikin, C., Gerlt, J.A., Almo, S.C.To be published.