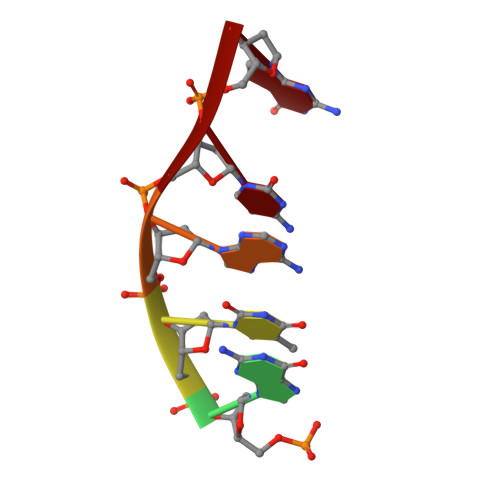
Structure of 9-amino-[N-(2-dimethylamino)propyl]acridine-4-carboxamide bound to d(CGTACG)(2): a comparison of structures of d(CGTACG)(2) complexed with intercalatorsin the presence of cobalt.
Adams, A., Guss, J.M., Denny, W.A., Wakelin, L.P.(2004) Acta Crystallogr D Biol Crystallogr 60: 823-828
- PubMed: 15103127
- DOI: https://doi.org/10.1107/S0907444904003907
- Primary Citation of Related Structures:
1RQY - PubMed Abstract:
The structure of the complex formed between 9-amino-[N-(2-dimethylamino)propyl]acridine-4-carboxamide and d(CGTACG)(2) has been refined to a resolution of 1.55 A. The complex crystallized in space group C222. An asymmetric unit comprises two strands of DNA, one disordered drug molecule, two cobalt(II) ions, two magnesium ions and 32 water molecules. The DNA helices stack in continuous columns, with their four central base pairs adopting a B-like motif. The terminal G.C base pairs engage in different interactions. At one end of the duplex there is a CpG dinucleotide overlap modified by ligand intercalation and terminal cytosine exchange between symmetry-related duplexes. An intercalation complex is formed involving four DNA duplexes, four disordered ligand molecules and two pairs of base tetrads. The other end of the DNA is frayed, with the terminal guanine lying in the minor groove of the next duplex in the column. The structure is stabilized by guanine N7-cobalt(II) coordination. The structure is compared with previously published isomorphous structures of d(CGTACG)(2) complexed with intercalators in the presence of cobalt and it is concluded that the formation of this crystal form is primarily determined by DNA-DNA interactions and packing forces, rather than by special interactions between the ligand and the DNA. Given the nature of the ligands found in these complexes, the relevance of the quadruplex structure to the biological activity of those agents, known to be topoisomerase poisons, is questioned.
- School of Molecular and Microbial Biosciences, University of Sydney, NSW 2006, Australia. a.adams@mmb.usyd.edu.au
Organizational Affiliation: