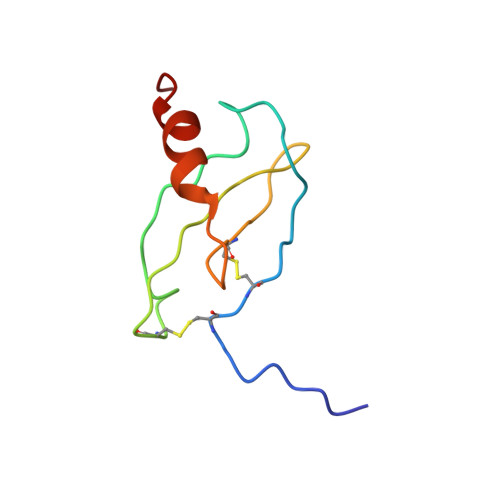
NMR structure of CXCR3 binding chemokine CXCL11 (ITAC).
Booth, V., Clark-Lewis, I., Sykes, B.D.(2004) Protein Sci 13: 2022-2028
- PubMed: 15273303
- DOI: https://doi.org/10.1110/ps.04791404
- Primary Citation of Related Structures:
1RJT - PubMed Abstract:
CXCL11 (ITAC) is one of three chemokines known to bind the receptor CXCR3, the two others being CXCL9 (Mig) and CXCL10 (IP-10). CXCL11 differs from the other CXCR3 ligands in both the strength and the particularities of its receptor interactions: It has a higher affinity, is a stronger agonist, and behaves differently when critical N-terminal residues are deleted. The structure of CXCL11 was determined using solution NMR to allow comparison with that of CXCL10 and help elucidate the source of the differences. CXCL11 takes on the canonical chemokine fold but exhibits greater conformational flexibility than has been observed for related chemokines under the same sample conditions. Unlike related chemokines such as IP-10 and IL-8, ITAC does not appear to form dimers at millimolar concentrations. The origin for this behavior can be found in the solution structure, which indicates a beta-bulge in beta-strand 1 that distorts the dimerization interface used by other CXC chemokines.
- Protein Engineering Network of Centres of Excellence, University of Alberta, Edmonton, Alberta, T5G 2S2, Canada.
Organizational Affiliation: