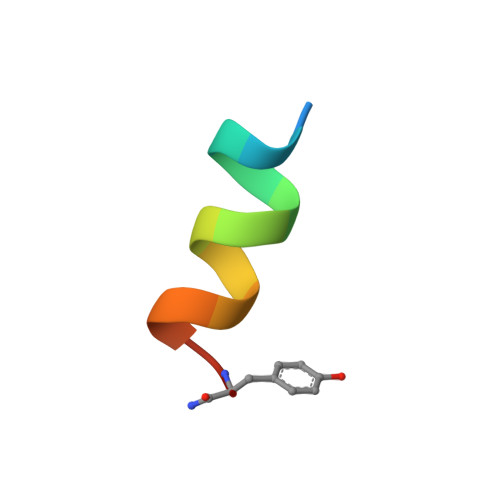
Helical structure and self-association in a 13 residue neuropeptide Y Y2 receptor agonist: relationship to biological activity.
Barnham, K.J., Catalfamo, F., Pallaghy, P.K., Howlett, G.J., Norton, R.S.(1999) Biochim Biophys Acta 1435: 127-137
- PubMed: 10561544
- DOI: https://doi.org/10.1016/s0167-4838(99)00214-9
- Primary Citation of Related Structures:
1QFA - PubMed Abstract:
The solution structure and self-association behaviour of a 13 residue peptide analogue of the C-terminal region of human neuropeptide Y (NPY) have been investigated. NMR analysis of Ac[Leu(28,31)]NPY(24-36), a potent Y2 receptor agonist, shows that it is unstructured in aqueous solution at 5-20 degrees C, but forms a well-defined helix (encompassing residues 25-35) in 40% trifluoroethanol/water at 20 degrees C. Sedimentation experiments show that, in contrast to many peptides in aqueous trifluoroethanol, Ac[Leu(28,31)]NPY(24-36) associates to form a trimer or, more likely, a tetramer in 40% trifluoroethanol, even though it is monomeric in water. This is consistent with the observation of inter-molecular nuclear Overhauser enhancements in trifluoroethanol. Possible models of the associated form that are consistent with the NMR data are described. The relevance of the helical structure observed in trifluoroethanol to the structure of this peptide bound to the NPY Y2 receptor is discussed.
- Biomolecular Research Institute, 343 Royal Parade, Parkville, Vic., Australia.
Organizational Affiliation: