Structure of the human 25 kDa FK506 binding protein complexed with rapamycin.
Liang, J., Hung, D.T., Schreiber, S.L., Clardy, J.(1996) J Am Chem Soc 118: 1231-1232
Experimental Data Snapshot
(1996) J Am Chem Soc 118: 1231-1232
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| FKBP25 | 116 | Homo sapiens | Mutation(s): 0 Gene Names: HUMAN HIPPOCAMPAL CDNA LIBRARY EC: 5.2.1.8 | 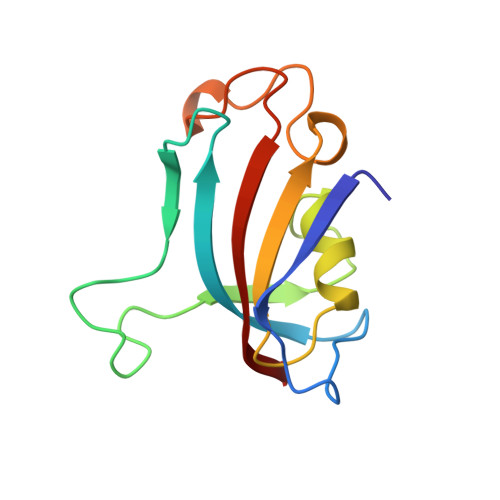 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q00688 (Homo sapiens) Explore Q00688 Go to UniProtKB: Q00688 | |||||
PHAROS: Q00688 GTEx: ENSG00000100442 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q00688 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| RAP Query on RAP | B [auth A] | RAPAMYCIN IMMUNOSUPPRESSANT DRUG C51 H79 N O13 QFJCIRLUMZQUOT-HPLJOQBZSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 86 | α = 90 |
| b = 86 | β = 90 |
| c = 55.6 | γ = 90 |
| Software Name | Purpose |
|---|---|
| X-PLOR | model building |
| X-PLOR | refinement |
| DENZO | data reduction |
| X-PLOR | phasing |