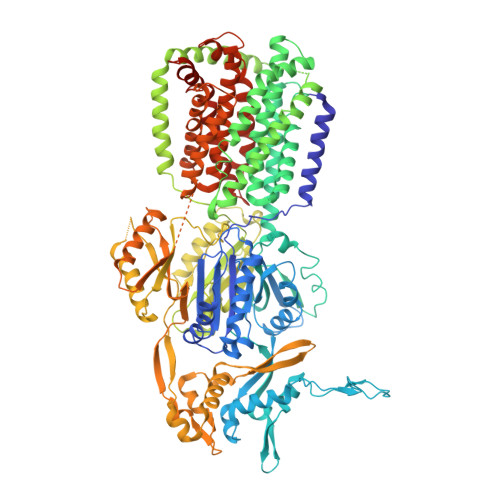
Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump.
Yu, E.W., McDermott, G., Zgurskaya, H.I., Nikaido, H., Koshland, D.E.(2003) Science 300: 976-980
- PubMed: 12738864
- DOI: https://doi.org/10.1126/science.1083137
- Primary Citation of Related Structures:
1OY6, 1OY8, 1OY9, 1OYD, 1OYE - PubMed Abstract:
Multidrug efflux pumps cause serious problems in cancer chemotherapy and treatment of bacterial infections. Yet high-resolution structures of ligand transporter complexes have previously been unavailable. We obtained x-ray crystallographic structures of the trimeric AcrB pump from Escherichia coli with four structurally diverse ligands. The structures show that three molecules of ligands bind simultaneously to the extremely large central cavity of 5000 cubic angstroms, primarily by hydrophobic, aromatic stacking and van der Waals interactions. Each ligand uses a slightly different subset of AcrB residues for binding. The bound ligand molecules often interact with each other, stabilizing the binding.
- Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720-3202, USA.
Organizational Affiliation: