Impact of Fc Glycans on The Effector Functions Vary with the Antibody mechanism of Action
Raju, T.S., Mulkerrin, M.G., Parker, M., De Vos, A.M., Gazzano-Santoro, H., Totpal, K., Ultsch, M.H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| immunoglobulin gamma-1 heavy chain constant region | 212 | Homo sapiens | Mutation(s): 0 | 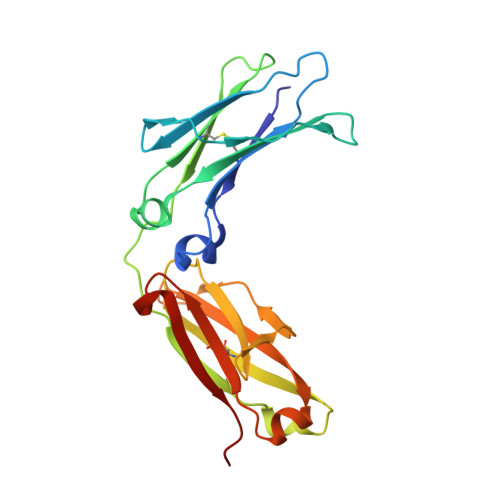 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01857 (Homo sapiens) Explore P01857 Go to UniProtKB: P01857 | |||||
PHAROS: P01857 GTEx: ENSG00000211896 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01857 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P01857-2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein A Z34C | 34 | synthetic construct | Mutation(s): 0 | 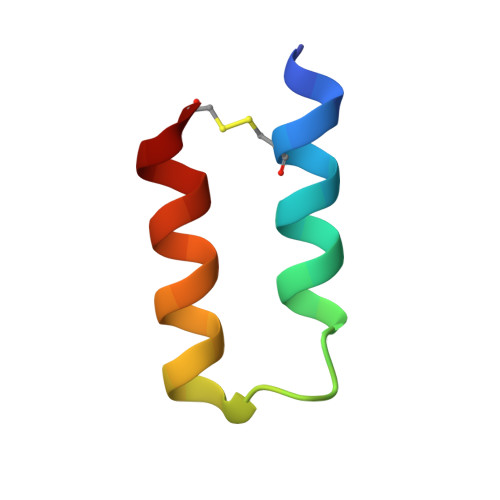 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[beta-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | E | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G31616YD GlyCosmos: G31616YD GlyGen: G31616YD | |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| alpha-D-mannopyranose-(1-3)-[beta-D-mannopyranose-(1-6)]beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | F | 6 |  | N-Glycosylation | |
Glycosylation Resources | |||||
GlyTouCan: G18521RG GlyCosmos: G18521RG GlyGen: G18521RG | |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 87.43 | α = 90 |
| b = 125.68 | β = 90 |
| c = 53.64 | γ = 90 |
| Software Name | Purpose |
|---|---|
| X-PLOR | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |