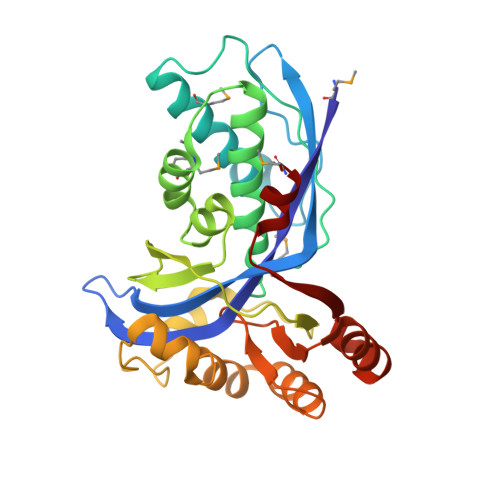
Biosynthesis of Isoprenoids: Crystal Structure of 4-Diphosphocytidyl-2C-Methyl-D-Erythritol Kinase
Miallau, L., Alphey, M.S., Kemp, L.E., Leonard, G.A., Mcsweeney, S.M., Hecht, S., Bacher, A., Eisenreich, W., Rohdich, F., Hunter, W.N.(2003) Proc Natl Acad Sci U S A 100: 9173
- PubMed: 12878729
- DOI: https://doi.org/10.1073/pnas.1533425100
- Primary Citation of Related Structures:
1OJ4 - PubMed Abstract:
4-Diphosphocytidyl-2C-methyl-d-erythritol kinase, an essential enzyme in the nonmevalonate pathway of isopentenyl diphosphate and dimethylallyl diphosphate biosynthesis, catalyzes the single ATP-dependent phosphorylation stage affording 4-diphosphocytidyl-2C-methyl-d-erythritol-2-phosphate. The 2-A resolution crystal structure of the Escherichia coli enzyme in a ternary complex with substrate and a nonhydrolyzable ATP analogue reveals the molecular determinants of specificity and catalysis. The enzyme subunit displays the alpha/beta fold characteristic of the galactose kinase/homoserine kinase/mevalonate kinase/phosphomevalonate kinase superfamily, arranged into cofactor and substrate-binding domains with the catalytic center positioned in a deep cleft between domains. Comparisons with related members of this superfamily indicate that the core regions of each domain are conserved, whereas there are significant differences in the substrate-binding pockets. The nonmevalonate pathway is essential in many microbial pathogens and distinct from the mevalonate pathway used by mammals. The high degree of sequence conservation of the enzyme across bacterial species suggests similarities in structure, specificity, and mechanism. Our model therefore provides an accurate template to facilitate the structure-based design of broad-spectrum antimicrobial agents.
- Division of Biological Chemistry and Molecular Microbiology, School of Life Sciences, University of Dundee, Dundee DD1 5EH, United Kingdom.
Organizational Affiliation: