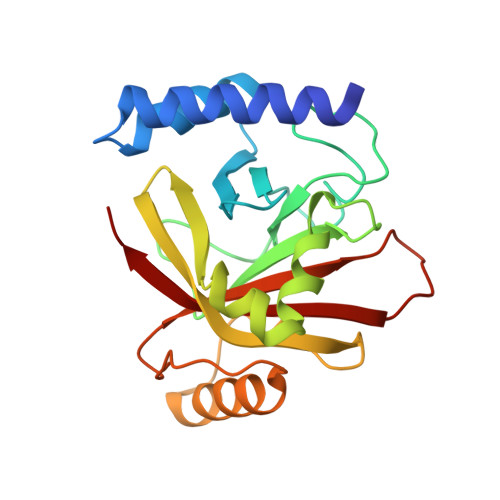
Structures of sortase B from Staphylococcus aureus and Bacillus anthracis reveal catalytic amino acid triad in the active site.
Zhang, R., Wu, R., Joachimiak, G., Mazmanian, S.K., Missiakas, D.M., Gornicki, P., Schneewind, O., Joachimiak, A.(2004) Structure 12: 1147-1156
- PubMed: 15242591
- DOI: https://doi.org/10.1016/j.str.2004.06.001
- Primary Citation of Related Structures:
1NG5, 1RZ2 - PubMed Abstract:
Surface proteins attached by sortases to the cell wall envelope of bacterial pathogens play important roles during infection. Sorting and attachment of these proteins is directed by C-terminal signals. Sortase B of S. aureus recognizes a motif NPQTN, cleaves the polypeptide after the Thr residue, and attaches the protein to pentaglycine cross-bridges. Sortase B of B. anthracis is thought to recognize the NPKTG motif, and attaches surface proteins to m-diaminopimelic acid cross-bridges. We have determined crystal structure of sortase B from B. anthracis and S. aureus at 1.6 and 2.0 A resolutions, respectively. These structures show a beta-barrel fold with alpha-helical elements on its outside, a structure thus far exclusive to the sortase family. A putative active site located on the edge of the beta-barrel is comprised of a Cys-His-Asp catalytic triad and presumably faces the bacterial cell surface. A putative binding site for the sorting signal is located nearby.
- Structural Biology Center and Midwest Center for Structural Genomics, Argonne National Laboratory, 9700 South Cass Avenue, Building 202, Argonne, IL 60439 USA.
Organizational Affiliation: