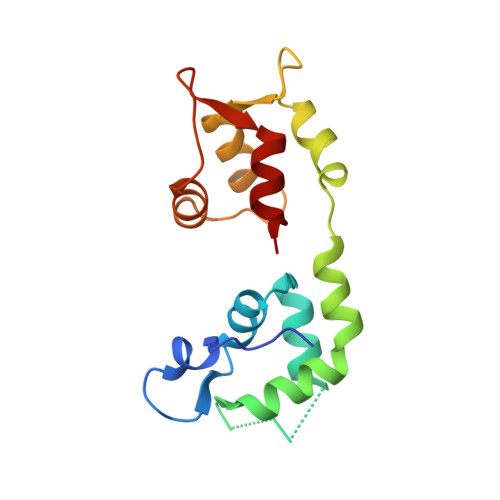
Two distinct myosin light chain structures are induced by specific variations within the bound IQ motifs-functional implications
Terrak, M., Wu, G., Stafford, W.F., Lu, R.C., Dominguez, R.(2003) EMBO J 22: 362-371
- PubMed: 12554638
- DOI: https://doi.org/10.1093/emboj/cdg058
- Primary Citation of Related Structures:
1M45, 1M46 - PubMed Abstract:
IQ motifs are widespread in nature. Mlc1p is a calmodulin-like myosin light chain that binds to IQ motifs of a class V myosin, Myo2p, and an IQGAP-related protein, Iqg1p, playing a role in polarized growth and cytokinesis in Saccharomyces cerevisiae. The crystal structures of Mlc1p bound to IQ2 and IQ4 of Myo2p differ dramatically. When bound to IQ2, Mlc1p adopts a compact conformation in which both the N- and C-lobes interact with the IQ motif. However, in the complex with IQ4, the N-lobe no longer interacts with the IQ motif, resulting in an extended conformation of Mlc1p. The two light chain structures relate to two distinct subfamilies of IQ motifs, one of which does not interact with the N-lobes of calmodulin-like light chains. The correlation between light chain structure and IQ sequence is demonstrated further by sedimentation velocity analysis of complexes of Mlc1p with IQ motifs from Myo2p and Iqg1p. The resulting 'free' N-lobes of myosin light chains in the extended conformation could mediate the formation of ternary complexes during protein localization and/or partner recruitment.
- Boston Biomedical Research Institute, 64 Grove Street, Watertown, MA 02472, USA.
Organizational Affiliation: