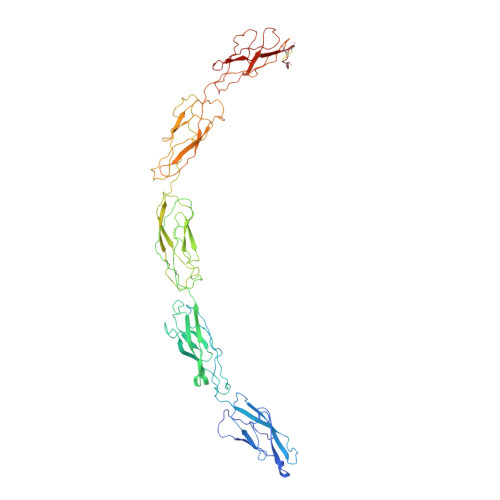
C-cadherin ectodomain structure and implications for cell adhesion mechanisms
Boggon, T.J., Murray, J., Chappuis-Flament, S., Wong, E., Gumbiner, B.M., Shapiro, L.(2002) Science 296: 1308-1313
- PubMed: 11964443
- DOI: https://doi.org/10.1126/science.1071559
- Primary Citation of Related Structures:
1L3W - PubMed Abstract:
Cadherins are transmembrane proteins that mediate adhesion between cells in the solid tissues of animals. Here we present the 3.1 angstrom resolution crystal structure of the whole, functional extracellular domain from C-cadherin, a representative "classical" cadherin. The structure suggests a molecular mechanism for adhesion between cells by classical cadherins, and it provides a new framework for understanding both cis (same cell) and trans (juxtaposed cell) cadherin interactions. The trans adhesive interface is a twofold symmetric interaction defined by a conserved tryptophan side chain at the membrane-distal end of a cadherin molecule from one cell, which inserts into a hydrophobic pocket at the membrane-distal end of a cadherin molecule from the opposing cell.
- Department of Biochemistry, Columbia University College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032, USA.
Organizational Affiliation: