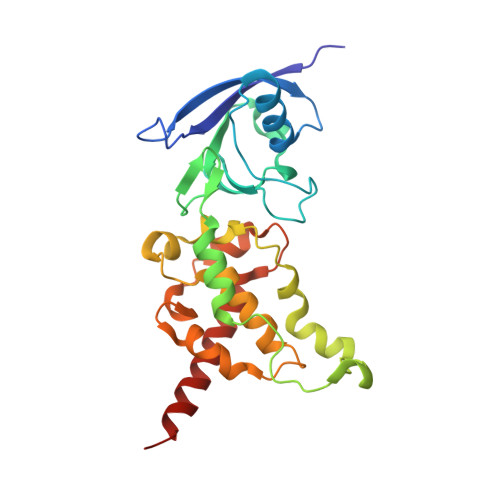
Crystallographic studies of the Escherichia coli quinol-fumarate reductase with inhibitors bound to the quinol-binding site.
Iverson, T.M., Luna-Chavez, C., Croal, L.R., Cecchini, G., Rees, D.C.(2002) J Biological Chem 277: 16124-16130
- PubMed: 11850430
- DOI: https://doi.org/10.1074/jbc.M200815200
- Primary Citation of Related Structures:
1KF6, 1KFY, 1L0V - PubMed Abstract:
The quinol-fumarate reductase (QFR) respiratory complex of Escherichia coli is a four-subunit integral-membrane complex that catalyzes the final step of anaerobic respiration when fumarate is the terminal electron acceptor. The membrane-soluble redox-active molecule menaquinol (MQH(2)) transfers electrons to QFR by binding directly to the membrane-spanning region. The crystal structure of QFR contains two quinone species, presumably MQH(2), bound to the transmembrane-spanning region. The binding sites for the two quinone molecules are termed Q(P) and Q(D), indicating their positions proximal (Q(P)) or distal (Q(D)) to the site of fumarate reduction in the hydrophilic flavoprotein and iron-sulfur protein subunits. It has not been established whether both of these sites are mechanistically significant. Co-crystallization studies of the E. coli QFR with the known quinol-binding site inhibitors 2-heptyl-4-hydroxyquinoline-N-oxide and 2-[1-(p-chlorophenyl)ethyl] 4,6-dinitrophenol establish that both inhibitors block the binding of MQH(2) at the Q(P) site. In the structures with the inhibitor bound at Q(P), no density is observed at Q(D), which suggests that the occupancy of this site can vary and argues against a structurally obligatory role for quinol binding to Q(D). A comparison of the Q(P) site of the E. coli enzyme with quinone-binding sites in other respiratory enzymes shows that an acidic residue is structurally conserved. This acidic residue, Glu-C29, in the E. coli enzyme may act as a proton shuttle from the quinol during enzyme turnover.
- Division of Chemistry and Chemical Engineering, Howard Hughes Medical Institute, California Institute of Technology, Pasadena, California 91125, USA.
Organizational Affiliation: