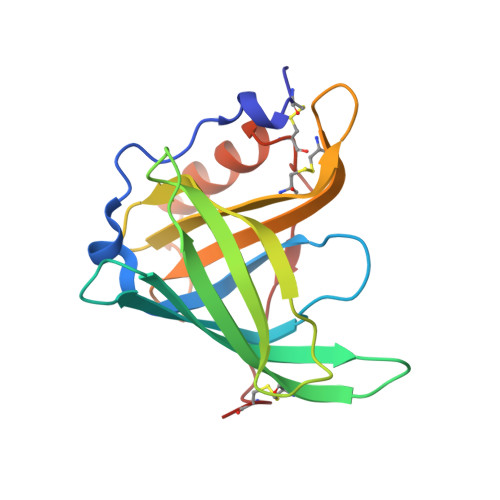
Structure of Chicken plasma retinol-binding protein
Zanotti, G., Calderone, V., Beda, M., Malpeli, G., Folli, C., Berni, R.(2001) Biochim Biophys Acta 1550: 64-69
- PubMed: 11738088
- DOI: https://doi.org/10.1016/s0167-4838(01)00268-0
- Primary Citation of Related Structures:
1IIU - PubMed Abstract:
The crystal structure of the specific carrier of retinol (retinol-binding protein, RBP) purified from chicken plasma has been determined (space group P2(1)2(1)2(1), with a=46.06(5) A, b=53.56(6) A, c=73.41(8) A, and one protein molecule in the asymmetric unit). Despite being obtained from a species phylogenetically distant from mammals, chicken holoRBP has an overall structure that closely resembles the previously determined structures of mammalian holoRBPs. The lack in chicken RBP of eight carboxy-terminal amino acid residues characteristic of mammalian RBPs does not significantly affect the protein structure. A distinctive feature of the avian protein is a better definition of the loop 63-67, close to the opening of the beta-barrel cavity accommodating the retinol molecule, which is rather disordered in the structures of mammalian RBPs.
- Department of Organic Chemistry, University of Padua and Biopolymer Research Center, CNR, Padua, Italy. giuseppe.zanotti@unipd.it
Organizational Affiliation: