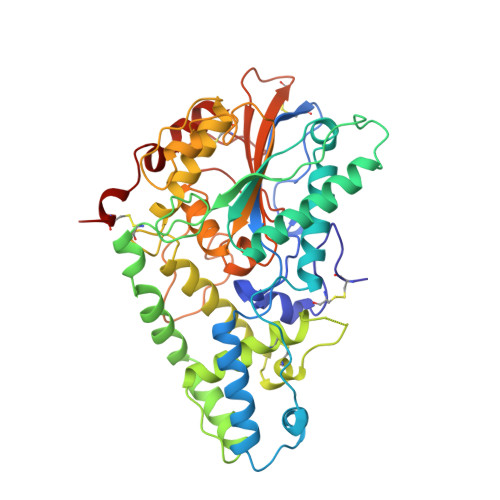
Crystal structure of phytase from Aspergillus ficuum at 2.5 A resolution.
Kostrewa, D., Gruninger-Leitch, F., D'Arcy, A., Broger, C., Mitchell, D., van Loon, A.P.(1997) Nat Struct Biol 4: 185-190
- PubMed: 9164457
- DOI: https://doi.org/10.1038/nsb0397-185
- Primary Citation of Related Structures:
1IHP - PubMed Abstract:
Phytase is a high molecular weight acid phosphatase. The structure has an alpha/beta-domain similar to that of rat acid phosphatase and an alpha-domain with a new fold.