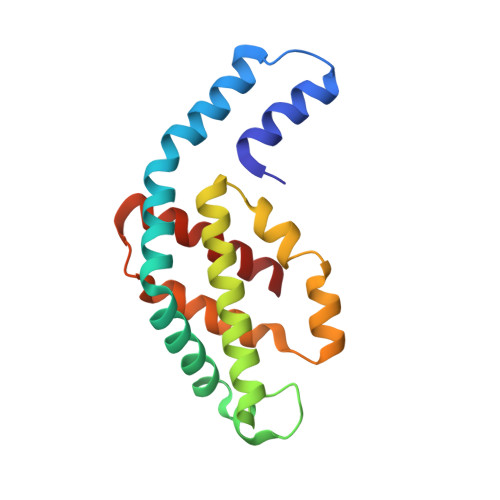
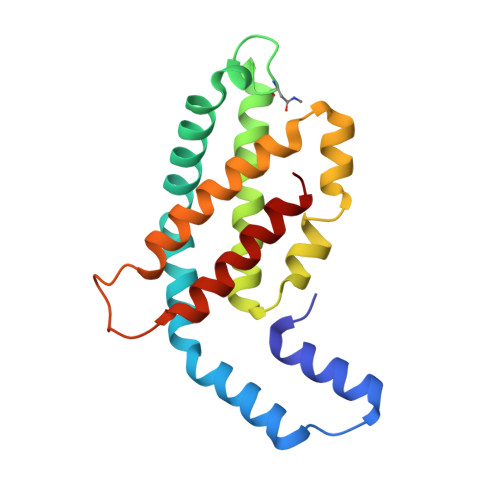
Structure of c-phycocyanin from the thermophilic cyanobacterium Synechococcus vulcanus at 2.5 A: structural implications for thermal stability in phycobilisome assembly.
Adir, N., Dobrovetsky, Y., Lerner, N.(2001) J Mol Biology 313: 71-81
- PubMed: 11601847
- DOI: https://doi.org/10.1006/jmbi.2001.5030
- Primary Citation of Related Structures:
1I7Y - PubMed Abstract:
The crystal structure of the light-harvesting phycobiliprotein, c-phycocyanin from the thermophilic cyanobacterium Synechochoccus vulcanus has been determined by molecular replacement to 2.5 A resolution. The crystal belongs to space group R32 with cell parameters a=b=188.43 A, c=61.28 A, alpha=beta=90 degrees, gamma=120 degrees, with one (alphabeta) monomer in the asymmetric unit. The structure has been refined to a crystallographic R factor of 20.2 % (R-free factor is 24.4 %), for all data to 2.5 A. The crystals were grown from phycocyanin (alphabeta)(3) trimers that form (alphabeta)(6) hexamers in the crystals, in a fashion similar to other phycocyanins. Comparison of the primary, tertiary and quaternary structures of the S. vulcanus phycocyanin structure with phycocyanins from both the mesophilic Fremyella diplsiphon and the thermophilic Mastigocladus laminosus were performed. We show that each level of assembly of oligomeric phycocyanin, which leads to the formation of the phycobilisome structure, can be stabilized in thermophilic organisms by amino acid residue substitutions. Each substitution can form additional ionic interactions at critical positions of each association interface. In addition, a significant shift in the position of ring D of the B155 phycocyanobilin cofactor in the S. vulcanus phycocyanin, enables the formation of important polar interactions at both the (alphabeta) monomer and (alphabeta)(6) hexamer association interfaces.
- Department of Chemistry and Institute of Catalysis, Science and Technology, Technion - Israel Institute of Technology, Technion City, Haifa 32000, Israel. nadir@tx.technion.ac.il
Organizational Affiliation: