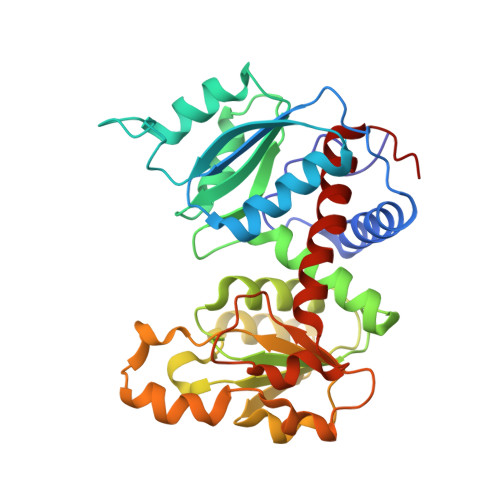
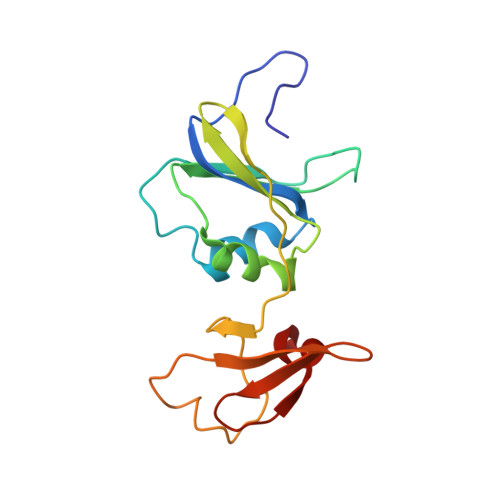
Direct structural evidence for a concerted allosteric transition in Escherichia coli aspartate transcarbamoylase.
Macol, C.P., Tsuruta, H., Stec, B., Kantrowitz, E.R.(2001) Nat Struct Biol 8: 423-426
- PubMed: 11323717
- DOI: https://doi.org/10.1038/87582
- Primary Citation of Related Structures:
1I5O - PubMed Abstract:
Regulation of protein function, often achieved by allosteric mechanisms, is central to normal physiology and cellular processes. Although numerous models have been proposed to account for the cooperative binding of ligands to allosteric proteins and enzymes, direct structural support has been lacking. Here, we used a combination of X-ray crystallography and small angle X-ray scattering in solution to provide direct structural evidence that the binding of ligand to just one of the six active sites of Escherichia coli aspartate transcarbamoylase induces a concerted structural transition from the T to the R state.
- Department of Chemistry, Boston College, Merkert Chemistry Center, Chestnut Hill, Massachusetts 02467, USA.
Organizational Affiliation: