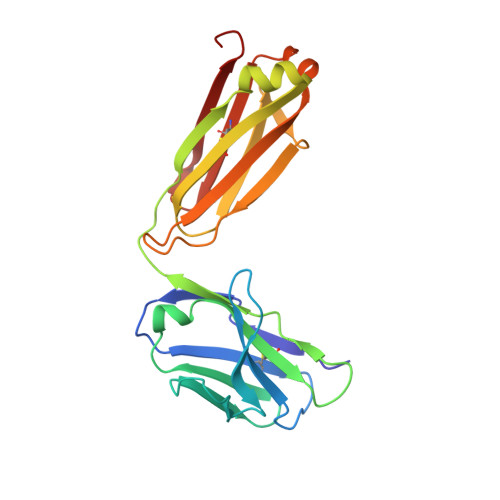
Evolutionary Transition Pathways for Changing Peptide Ligand Specificity and Structure
Hoffmuller, U., Knaute, T., Hahn, M., Hohne, W., Schneider-Mergener, J., Kramer, A.(2000) EMBO J 19: 4866
- PubMed: 10990450
- DOI: https://doi.org/10.1093/emboj/19.18.4866
- Primary Citation of Related Structures:
1HH6, 1HH9 - PubMed Abstract:
We identified evolutionary pathways for the inter- conversion of three sequentially and structurally unrelated peptides, GATPEDLNQKL, GLYEWGGARI and FDKEWNLIEQN, binding to the same site of the hypervariable region of the anti-p24 (HIV-1) monoclonal antibody CB4-1. Conversion of these peptides into each other could be achieved in nine or 10 single amino acid substitution steps without loss of antibody binding. Such pathways were identified by analyzing all 7 620 480 pathways connecting 2560 different peptides, and testing them for CB4-1 binding. The binding modes of intermediate peptides of selected optimal pathways were characterized using complete sets of substitution analogs, revealing that a number of sequential substitutions accumulated without changing the pattern of key interacting residues. At a distinct step, however, one single amino acid exchange induces a sudden change in the binding mode, indicating a flip in specificity and conformation. Our data represent a model of how different specificities, structures and functions might evolve in protein-protein recognition.
- Institut für Medizinische Immunologie and Institut für Biochemie, Universitätsklinikum Charité, Humboldt-Universität zu Berlin, Schumannstrasse 20/21, 10098 Berlin, Germany.
Organizational Affiliation: