Crystal Structure of a Carboxy-Terminal Fragment of Growth-Arrest-Specific Protein Gas6: Receptor Tyrosine Kinase Activation by Laminin G-Like Domains
Sasaki, T., Knyazev, P.G., Cheburkin, Y., Gohring, W., Tisi, D., Ullrich, A., Timpl, R., Hohenester, E.(2002) J Biological Chem 277: 44164
- PubMed: 12218057
- DOI: https://doi.org/10.1074/jbc.M207340200
- Primary Citation of Related Structures:
1H30 - PubMed Abstract:
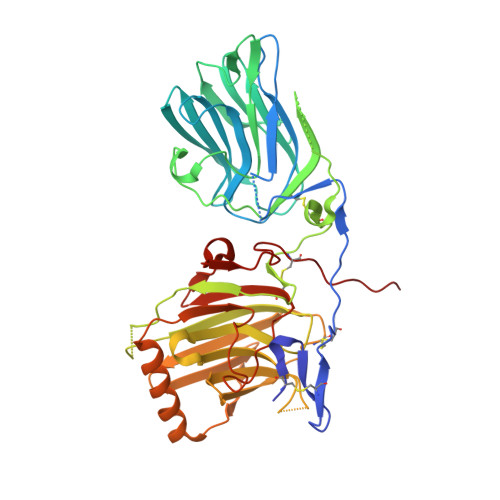
Receptor tyrosine kinases of the Axl family are activated by Gas6, the product of growth arrest-specific gene 6. Gas6-Axl signaling is implicated in cell survival, adhesion, and migration. The receptor-binding site of Gas6 is located within a C-terminal pair of laminin G-like (LG) domains that do not resemble any other receptor tyrosine kinase ligand. We report the crystal structure at 2.2-A resolution of a Gas6 fragment spanning both LG domains (Gas6-LG). The structure reveals a V-shaped arrangement of LG domains strengthened by an interdomain calcium-binding site. LG2 of Gas6-LG contains two unusual features: an alpha-helix cradled by one edge of the LG beta-sandwich and a conspicuous patch of surface-exposed hydrophobic residues. Mutagenesis of some residues in this patch reduces Gas6-LG binding to the extracellular domain of Axl as well as Axl activation in glioblastoma cells, identifying a component of the receptor-binding site of Gas6.
- Max-Planck-Institut für Biochemie, D-82152 Martinsried, Germany.
Organizational Affiliation: