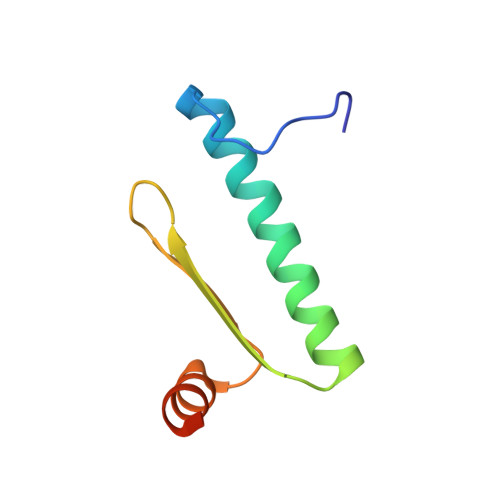
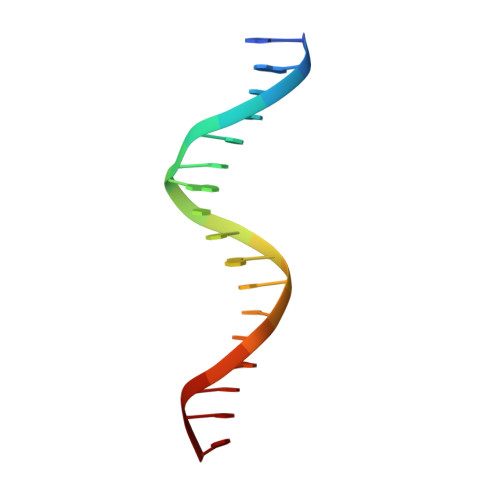
Crystal structure of MEF2A core bound to DNA at 1.5 A resolution.
Santelli, E., Richmond, T.J.(2000) J Mol Biology 297: 437-449
- PubMed: 10715212
- DOI: https://doi.org/10.1006/jmbi.2000.3568
- Primary Citation of Related Structures:
1EGW - PubMed Abstract:
Members of the myocyte enhancer factor-2 (MEF2) family of transcription factors bind to and activate transcription through A+T-rich DNA sequences found primarily, but not exclusively, in the promoters of muscle-specific genes. Their importance has been established for myogenic development and in activation of the immediate-early gene, c-jun, and recently further functional roles in the immune system have emerged. The MEF2 factors belong to the MADS-box superfamily, sharing homology in a 58 amino acid domain that mediates DNA binding and dimerization. The structures of two MADS-box proteins, SRF and MCM1, bound to their cognate DNA have been previously reported and shown to share extensive similarity in their mode of DNA binding. We have solved the structure of MEF2A 2-78 bound to its DNA consensus sequence at 1.5 A resolution. It reveals how the absence of amino acids N-terminal to the MADS-box contributes to the DNA binding properties of MEF2 proteins and shows that the MEF domain C-terminal to the MADS-box adopts a conformation considerably different from the same region in SRF and MCM1.
- Institut für Molekularbiologie und Biophysik, ETH Zurich, Zürich, CH, Switzerland.
Organizational Affiliation: