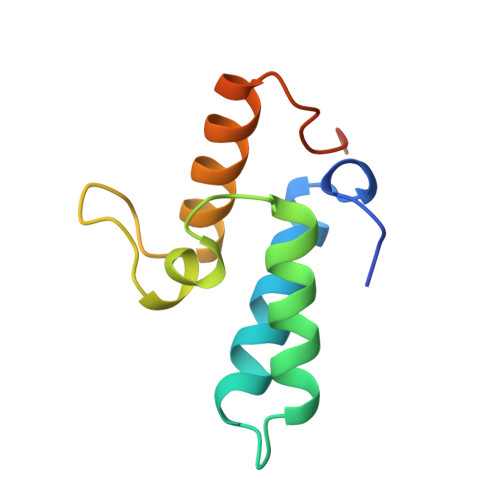
The crystal structure of the immunity protein of colicin E7 suggests a possible colicin-interacting surface.
Chak, K.F., Safo, M.K., Ku, W.Y., Hsieh, S.Y., Yuan, H.S.(1996) Proc Natl Acad Sci U S A 93: 6437-6442
- PubMed: 8692833
- DOI: https://doi.org/10.1073/pnas.93.13.6437
- Primary Citation of Related Structures:
1CEI - PubMed Abstract:
The immunity protein of colicin E7 (ImmE7) can bind specifically to the DNase-type colicin E7 and inhibit its bactericidal activity. Here we report the 1.8-angstrom crystal structure of the ImmE7 protein. This is the first x-ray structure determined in the superfamily of colicin immunity proteins. The ImmE7 protein consists of four antiparallel alpha-helices, folded in a topology similar to the architecture of a four-helix bundle structure. A region rich in acidic residues is identified. This negatively charged area has the greatest variability within the family of DNase-type immunity proteins; thus, it seems likely that this area is involved in specific binding to colicin. Based on structural, genetic, and kinetic data, we suggest that all the DNase-type immunity proteins, as well as colicins, share a "homologous-structural framework" and that specific interaction between a colicin and its cognate immunity protein relies upon how well these two proteins' charged residues match on the interaction surface, thus leading to specific immunity of the colicin.
- Institute of Biochemistry, National Yang-Ming University, Taipei, Taiwan, Republic of China.
Organizational Affiliation: