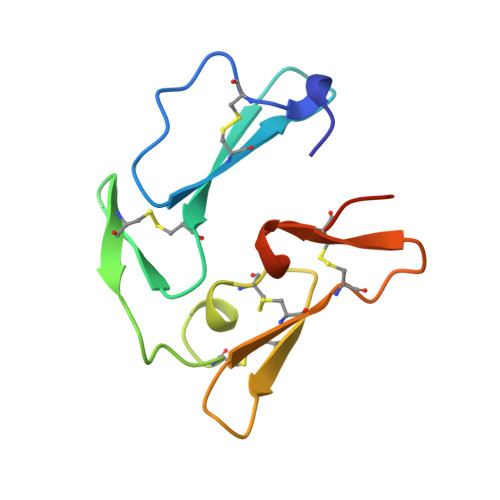
The crystal structure of C-terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate.
Chitarra, V., Holm, I., Bentley, G.A., Petres, S., Longacre, S.(1999) Mol Cell 3: 457-464
- PubMed: 10230398
- DOI: https://doi.org/10.1016/s1097-2765(00)80473-6
- Primary Citation of Related Structures:
1B9W - PubMed Abstract:
The C-terminal proteolytic processing product of merozoite surface protein 1 (MSP1) appears essential for successful erythrocyte invasion by the malarial parasite, Plasmodium. We have determined the crystal structure at 1.8 A resolution of a soluble baculovirus-recombinant form of the protein from P. cynomolgi, which confers excellent protective efficacy in primate vaccination trials. The structure comprises two EGF-like domains, and sequence comparisons strongly suggest that the same conformation is present in all species of Plasmodium, including P. falciparum and P. vivax, which are pathogenic in man. In particular, conserved interdomain contacts between the two EGF modules should preserve the compact form of the molecule in all species. Implications of the crystal structure for anti-malarial vaccine development are discussed.
- Unité d'Immunologie Structurale (CNRS URA 1961), Institut Pasteur, Paris, France.
Organizational Affiliation: