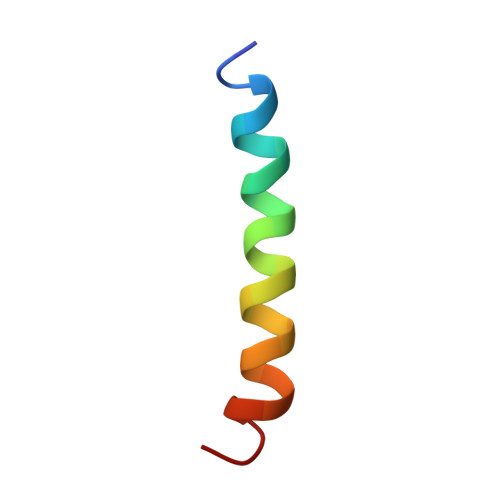
Solution structure of residues 1-28 of the amyloid beta-peptide.
Talafous, J., Marcinowski, K.J., Klopman, G., Zagorski, M.G.(1994) Biochemistry 33: 7788-7796
- PubMed: 7516706
- DOI: https://doi.org/10.1021/bi00191a006
- Primary Citation of Related Structures:
1AMB, 1AMC - PubMed Abstract:
The three-dimensional solution structure of residues 1-28 of the amyloid beta-peptide was determined using nuclear magnetic resonance spectroscopy, distance geometry, and molecular dynamic techniques. The nuclear magnetic resonance data used to derive the structure consisted of nuclear Overhauser enhancements, vicinal coupling constants, and temperature coefficients of the amide-NH chemical shifts. The beta-peptide is the major proteinaceous component of amyloid deposits in Alzheimer's disease. In membrane-like media, the peptide folds to form a predominately alpha-helical structure with a bend centered at residue 12. The side chains of histidine-13 and lysine-16 are close, residing on the same face of the helix. Their proximity may constitute a binding motif with the heparan sulfate proteoglycans. The molecular details of the structure shown here could facilitate the design of rational treatments to curtail the binding of heparan sulfate proteoglycans or to prevent an alpha-helix-->beta-sheet conversion that may occur during the early stages of amyloid formation in Alzheimer's disease.
- Department of Chemistry, Case Western Reserve University, Cleveland, Ohio 44106.
Organizational Affiliation: