High-Affinity Alkynyl Bisubstrate Inhibitors of NicotinamideN-Methyltransferase (NNMT).
Policarpo, R.L., Decultot, L., May, E., Kuzmic, P., Carlson, S., Huang, D., Chu, V., Wright, B.A., Dhakshinamoorthy, S., Kannt, A., Rani, S., Dittakavi, S., Panarese, J.D., Gaudet, R., Shair, M.D.(2019) J Med Chem 62: 9837-9873
- PubMed: 31589440
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01238
- Primary Citation of Related Structures:
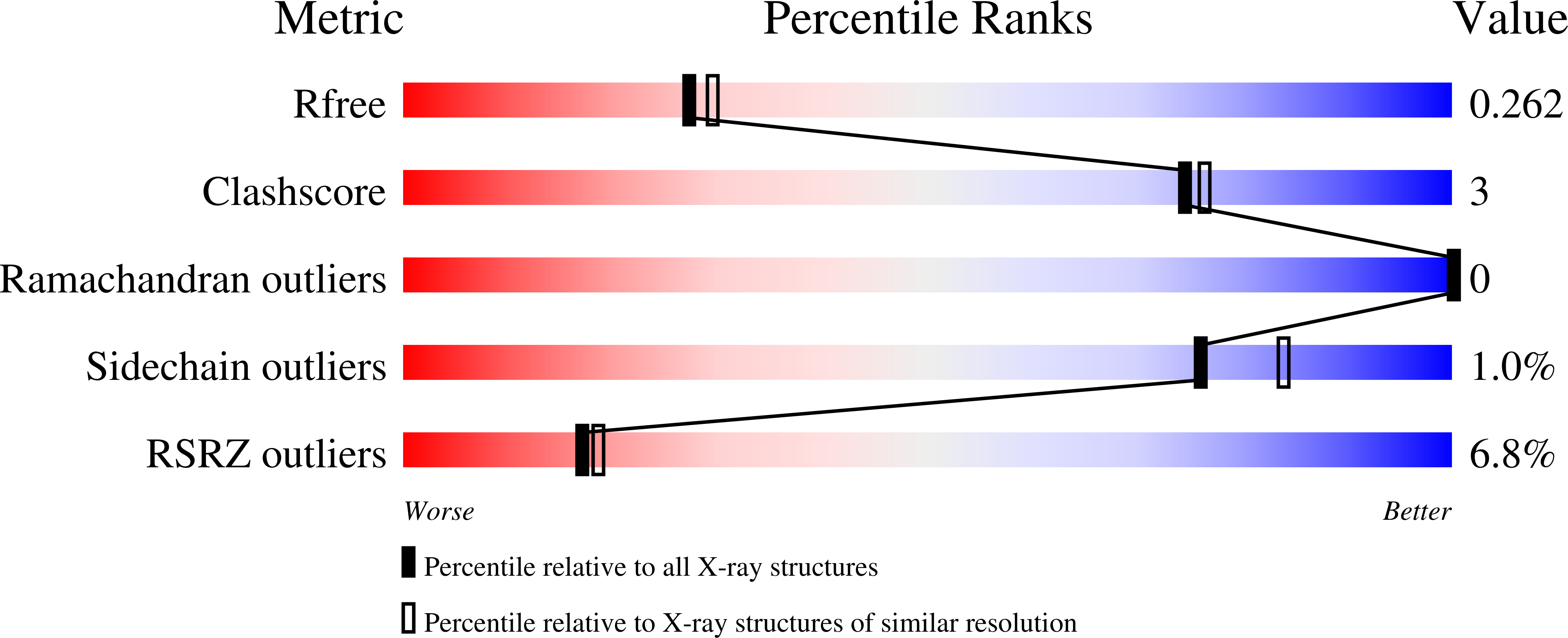
6ORR - PubMed Abstract:
Nicotinamide N -methyltransferase (NNMT) is a metabolic enzyme that methylates nicotinamide (NAM) using cofactor S -adenosylmethionine (SAM). NNMT overexpression has been linked to diabetes, obesity, and various cancers. In this work, structure-based rational design led to the development of potent and selective alkynyl bisubstrate inhibitors of NNMT. The reported nicotinamide-SAM conjugate (named NS1) features an alkyne as a key design element that closely mimics the linear, 180° transition state geometry found in the NNMT-catalyzed SAM → NAM methyl transfer reaction. NS1 was synthesized in 14 steps and found to be a high-affinity, subnanomolar NNMT inhibitor. An X-ray cocrystal structure and SAR study revealed the ability of an alkynyl linker to span the methyl transfer tunnel of NNMT with ideal shape complementarity. The compounds reported in this work represent the most potent and selective NNMT inhibitors reported to date. The rational design principle described herein could potentially be extended to other methyltransferase enzymes.
Organizational Affiliation:
BioKin Ltd. , Watertown , Massachusetts 02472 , United States.