Snapshots of the Catalytic Cycle of the Industrial Enzyme alpha-Amino-epsilon-Caprolactam Racemase (ACLR) Observed Using X-ray Crystallography
Frese, A., Sutton, P.W., Turkenburg, J.P., Grogan, G.(2017) ACS Catal 7: 1045-1048
Experimental Data Snapshot
(2017) ACS Catal 7: 1045-1048
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aminotransferase class-III | 440 | Rhizobium freirei PRF 81 | Mutation(s): 0 Gene Names: RHSP_08934 | 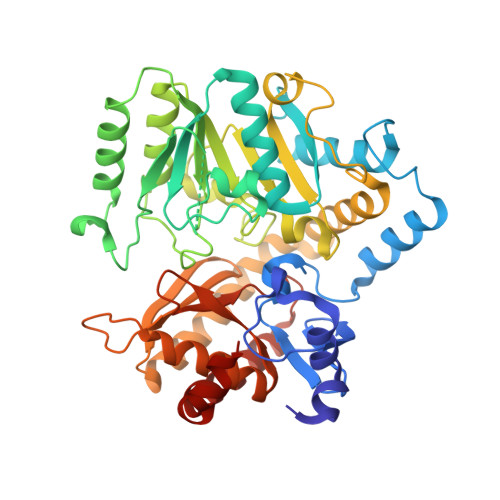 | |
UniProt | |||||
Find proteins for N6UXY4 (Rhizobium freirei PRF 81) Explore N6UXY4 Go to UniProtKB: N6UXY4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | N6UXY4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PLP Query on PLP | B [auth A] | PYRIDOXAL-5'-PHOSPHATE C8 H10 N O6 P NGVDGCNFYWLIFO-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | C [auth A], D [auth A], E [auth A], F [auth A], G [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 87.613 | α = 90 |
| b = 76.527 | β = 113.12 |
| c = 58.57 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| SCALA | data scaling |
| MOLREP | phasing |