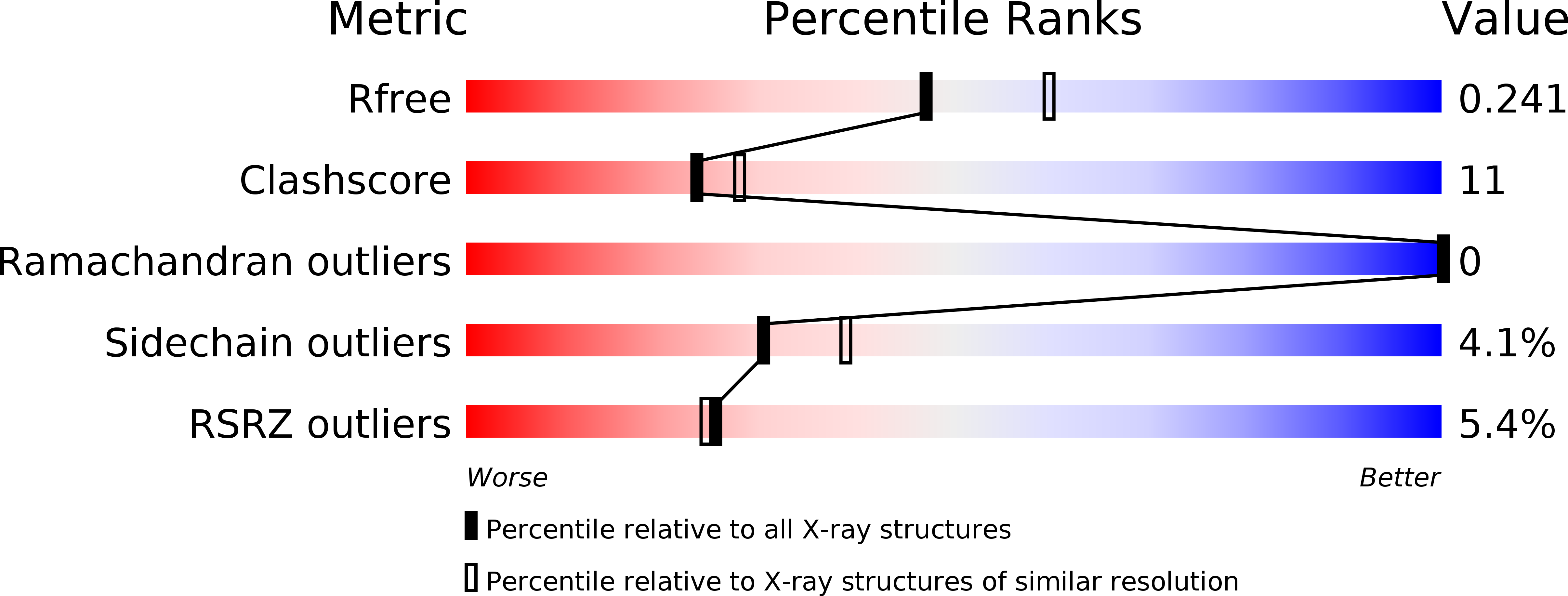
Baicalin inhibits the lethality of ricin in mice by inducing protein oligomerization.
Dong, J., Zhang, Y., Chen, Y., Niu, X., Zhang, Y., Li, R., Yang, C., Wang, Q., Li, X., Deng, X.(2015) J Biol Chem 290: 12899-12907
- PubMed: 25847243
- DOI: https://doi.org/10.1074/jbc.M114.632828
- Primary Citation of Related Structures:
4Q2V - PubMed Abstract:
Toxic ribosome-inactivating proteins abolish cell viability by inhibiting protein synthesis. Ricin, a member of these lethal proteins, is a potential bioterrorism agent. Despite the grave challenge posed by these toxins to public health, post-exposure treatment for intoxication caused by these agents currently is unavailable. In this study, we report the identification of baicalin extracted from Chinese herbal medicine as a compound capable of inhibiting the activity of ricin. More importantly, post-exposure treatment with baicalin significantly increased the survival of mice poisoned by ricin. We determined the mechanism of action of baicalin by solving the crystal structure of its complex with the A chain of ricin (RTA) at 2.2 Å resolution, which revealed that baicalin interacts with two RTA molecules at a novel binding site by hydrogen bond networks and electrostatic force interactions, suggesting its role as molecular glue of the RTA. Further biochemical and biophysical analyses validated the amino acids directly involved in binding the inhibitor, which is consistent with the hypothesis that baicalin exerts its inhibitory effects by inducing RTA to form oligomers in solution, a mechanism that is distinctly different from previously reported inhibitors. This work offers promising leads for the development of therapeutics against ricin and probably other ribosome-inactivating proteins.
Organizational Affiliation:
From the Key Laboratory of Zoonosis, Ministry of Education, Institute of Zoonosis, College of Veterinary Medicine, Jilin University, Changchun 130062, the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan 430223.